Oxygen Bomb Calorimetry
You will be measuring the heat of combustion, DH°c, for each of the two esters synthesized in the prior portion of the experiment.
The stoichiometry of the combustion is:
C8H14O2 + 21/2 O2 ---> 8CO2 + 7H2O
The difference between the two compounds should be (to a first approximation) the strain energy in the cyclopropane ring.
Q. Which of the two compounds will give off the most heat when combusted? Why?
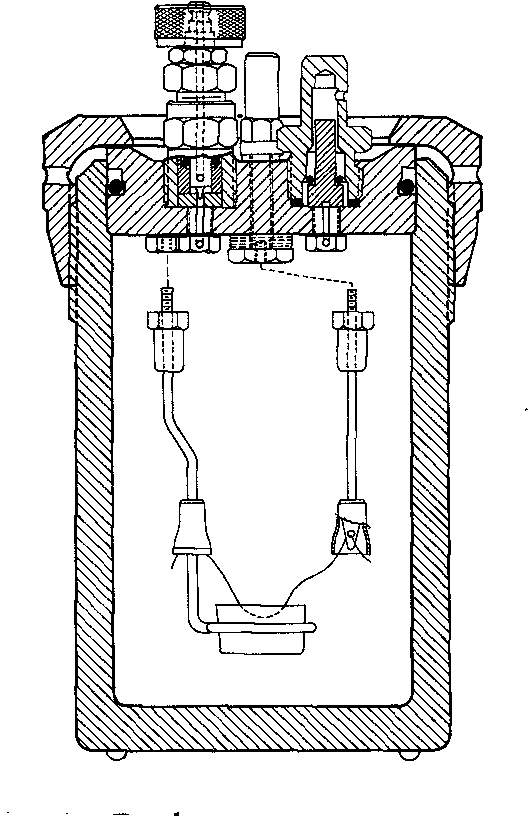
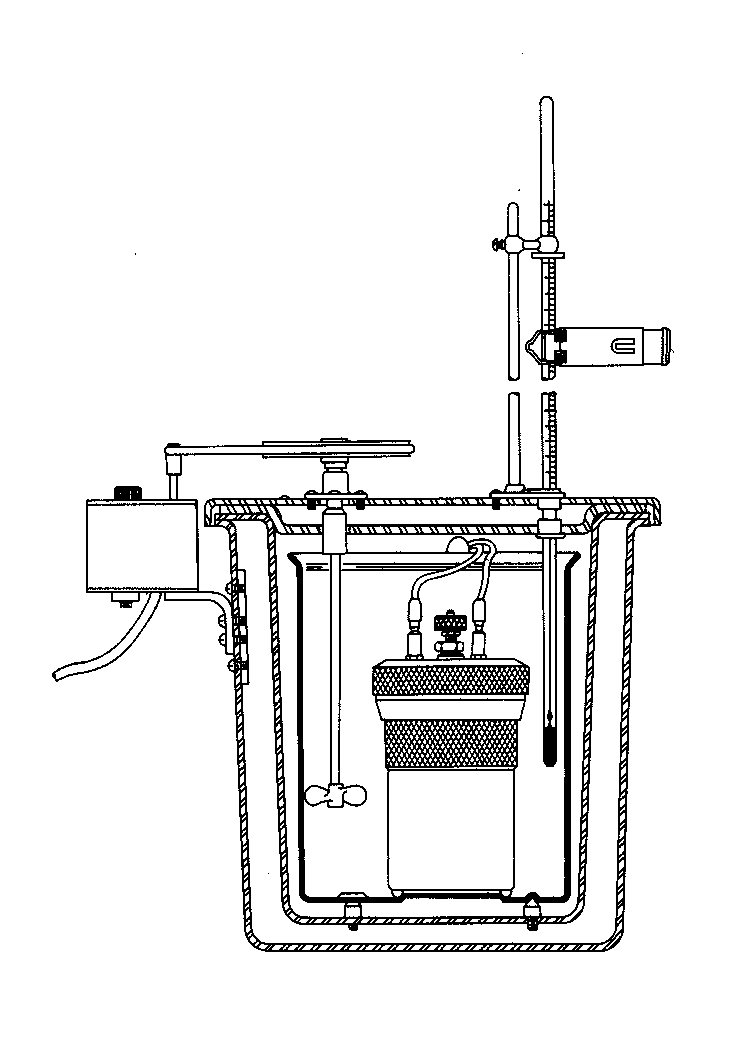
Diagram of the calorimeter apparatus.
 |
 |
| The bomb | The bomb and its associated water jacket, stirrer and thermometer |
The general procedure is to:
You should watch the slide presentation on use of the bomb calorimeter to become familiar with details of the procedure. We will try to get this info onto the Web page at some time in the future. For now, you may follow the links below to what we have.
Use of the Bomb Calorimeter
Finding the calorimeter constant (combustion of methylsalicylate)
Analysis of Data
Propagation of Error
Sample data: Excel format
Temperature correction spreadsheet files are posted here.
The thermometers you use are calibrated on the assumption that the entire mercury column is submerged in the liquid. Since you do not do this, contraction of the glass between the surface of the water bath and the top of the mercury introduces a systematic error. Proper use of the T correction in these files will remove that error.
Back to CH362 Home Page
02/09/2013-->
CP