(ref: Mohrig, Ch. 20, pp. 291-308)
Gas chromatography (or more properly, gas-liquid chromatography) relies on the sample components interacting with a liquid phase as a carrier gas sweeps over it. Weakly-interacting compounds (high vapor pressure/low boiling point, nonpolar) will have a shorter retention time than more strongly-interacting components of a mixture. The stationary phase (liquid) can be varied on the basis of chemical and physical interactions with the kinds of compounds present in a given mixture.
The instrument design

 (Source: Wikipedia)
(Source: Wikipedia)
The injector
The first stage is to introduce the sample. We use a syringe to introduce a tiny amount (1-3 μL) into the "injector" (a heated metal block) through a silicone septum:

The septum prevents air from getting in; the high temperature flash vaporizes the sample. The injector has carrier gas (helium) flowing through.
The column

A view of the two columns in our GC.
Our GCs have two columns: we will use the one labeled "B" (right) and the other winds up being a reference. The liquid phase (DC200, a polydimethylsilicone) is adsorbed on crushed firebrick (an inert ceramic like your porcelain drying plate) and packed into a steel or glass tube, which is then fit into the instrument. Upon vaporization of the sample, it is carried onto the column by the mobile phase carrier gas (usually He). There, it repeatedly absorbs and desorbs; the strength of the interaction with the stationary phase determines how long it takes to reach the end. That time is called the retention time.
Modern analytical GCs use a capillary column; this is an extremely thin, long glass tube coated (on the inside) with the stationary phase. This requires a special injector since only micrograms of sample can be accommodates (nanoliters). Sensitive detectors are also required.
The detector
Several kinds of detectors can be used:
- flame ionization detectors (FIDs) burn the sample and detect the ions & radicals formed in combustion
- Electron capture detectors fire electrons at the outflow and respond when something captures an electron (common for halogenated compounds)
- Mass spectrometers can provide a lot of information--the mass spectrum of anything coming off the column>/li>
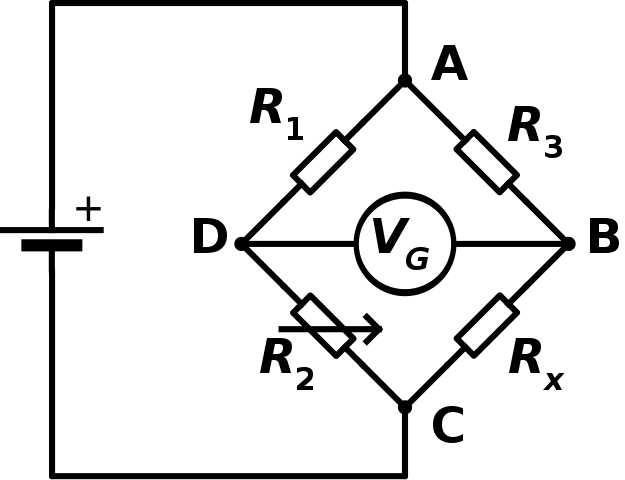
- Ours is a thermal conductivity detector, a very general detector based on a Wheatstone bridge:
 (Wikipedia)
(Wikipedia)
One of the resistors is a heated filament that lies in the path of the column effluent; as anything other than He carrier gas comes out, it conducts heat away from the filament in proportion to its heat capacity. The resistance of the filament changes, and a voltage is registered. The voltage is proportional to the mass and the heat capacity of the sample component coming off.
Interpreting the GC output
You need to note two major pieces of data from the recorder/integrator: - the retention time tells you what is coming off;
- The peak area (absolute, or as a percent) tells you how much sample you have in the mixture.
(You also need to know response factors, since compounds will have different heat capacities! Fortunately, the response factors for our compounds are close enough to being identical that we will disregard them. For careful analytical work, you must calibrate yourself by measuring response factors for standardized solutions of the target components.)

 (Source: Wikipedia)
(Source: Wikipedia)


 (Wikipedia)
(Wikipedia)