Important Disaccharides and Polysaccharides
Monosaccharides can be linked through glycosidic bonds between anomeric carbons and any of the hydroxyl groups on a second saccharide unit. Specific linkages can lead to very specific chemical and biological behavior.
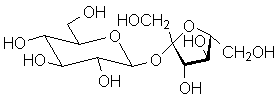
We can also have trioses, made up of three monosaccharide units; tetroses, made up of four monosaccharide units, etc. An example is "sialyl Lewisx," a tetrasaccharide that plays a key role in cellular recognition and immune response:
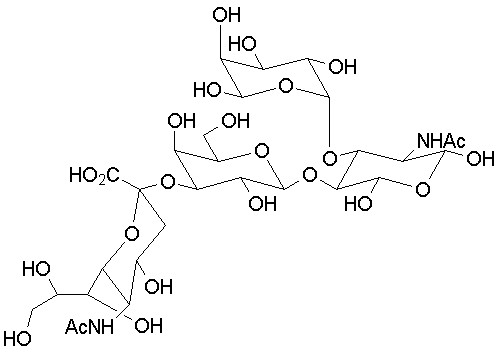 |
Blood typing antigens also involve molecular recognition of oligosaccharide units. The following structures are seen in, respectively, Type A, Type B and Type O blood (the link to the protein is more complex, but the terminal tri- or tetrasaccharide deterimines the function):
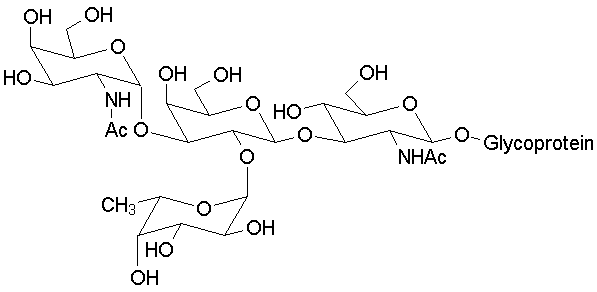 |
|
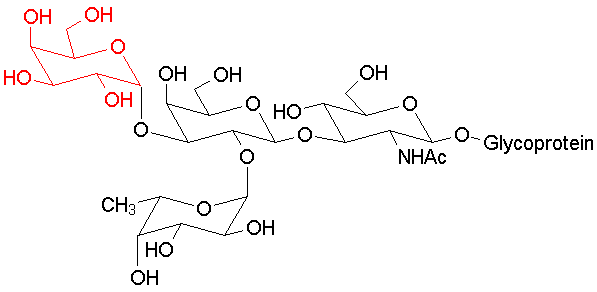 |
|
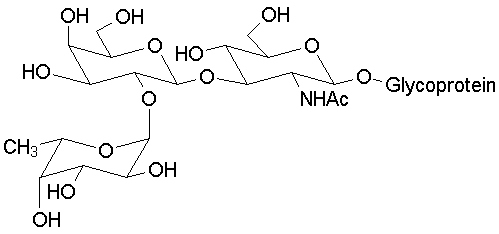 |
Now consider the polymeric forms.
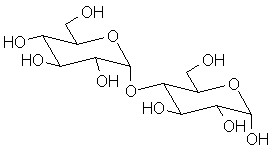
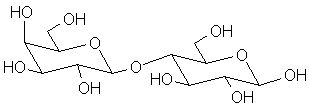
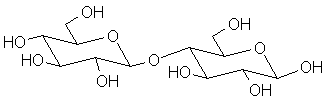
The most important polysaccharides are all composed primarily of glucose units. The difference between them is a combination of the amount of branching (one sugar donating 2 or more hydroxyls to glycosidic links) and the anomeric configuration.
Cellulose:
The major structural component of plants is a linear polymer linked almost entirely by b-links. This leads to a very regular structure that can crystallize easily (up to 80% of cellulose is crystalline) and is thus quite rigid, not water-soluble and resistant to chemical action. In plants, the cell wall is composed primarily of cellulose; other components include hemicellulose (a more branched form of cellulose that is less crystalline and more flexible) and lignin (oligomeric phenolic structures; obtained by dehydration and oxidation of saccharide units; these serve as potent antioxidants).
Starch:
Starch, on the other hand, is linked by a-linkages. The amylose portion is also a linear polymer, though it can branch somewhat more than cellulose. Amylopectin, another portion of strarch, is much more branched and is water-soluble. Starch is more readily hydrolyzed than cellulose and is thus a storage reservoir for glucose generated from photosynthesis.