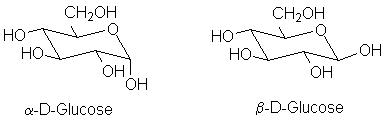The normal form of most sugars is in a cyclic hemiacetal form. The preferred form varies from sugar to sugar: some prefer to be a 6-member ring "pyranose", like glucose:
acetal.xyz
Backgrounds:
Black
White
Stop animation
Restart animation
Others, like ribose and fructose, prefer the 5-member ring "furanose" form.
An important aspect of hemiacetal formation is the production of a new stereocenter at the carbonyl carbon. This is called the anomeric position. In general, both possible isomers are formed. However, the two diastereomers can often be separated. The β-form of D-glucose crystallizes, but after redissolving the pure β form in water it reconverts to an equilibrium mixture of the two anomeric forms; this process is called mutarotation because it can be detected by a change in the optical rotation.