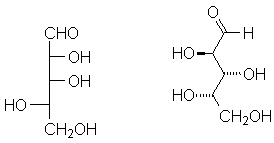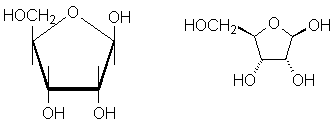The Fischer and Haworth Projections
The presence of multiple stereocenters in carbohydrates makes it a challenge to accurately describe the structure of such a molecule in any way that is reasonably simple. We need to accurately depict the absolute stereochemistry (R or S) as well as the relative arrangement of atoms in space. Two conventions have been developed to try to simplify the situation.
I. The Fischer Projection
The Fischer Projection is a convention to depict the correct configuration of a carbon as simply as possible. The four bonds to carbon are indicated with a cross; the carbon is in the plane of the paper, the horizontal bonds are coming out of the plane of the paper, and the vertical bonds are going into the plane of the paper:
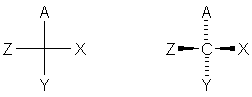
This is straightforward, just definition. Most students have the most difficulty when dealing with multiple stereocenters. Remember that each stereocenter in a Fischer projection is viewed separately. An example is below:
Rotate the structure on the left so that sequentially each carbon is the object of focus. Match it with the appropriate carbons in the Fischer projection (middle) and the wedge-and-dash structure (right). Can you assign R or S configurations to each stereocenter?
(Hydrogens may be shown or not; often the C-H bonds are indicated by drawing the bond line but omitting the symbol.)
We can also recognize that there are several ways to move substituents around in a Fischer projection and keep the same configuration. Rotating by 180° gives back the same structure. Exchanging any two positions gives the opposite configuration, but pairwise exchage of two sets gives back the same structure. Try making models to convince yourself:
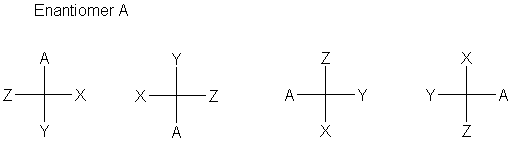 |
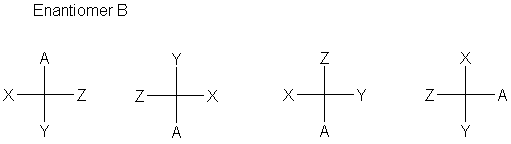 |
II. The Haworth Projection
As useful as the Fischer projection is (it is an excellent way to keep track of relative stereochemistry), it gives a poor sense of the real structure of carbohydrates. (See Hemiacetal Formation.) The Haworth projection is a way around this limitation that does not require you to try to convey the complete 3D image of the molecule.
We divide Haworth projections into two classes. 5-member ring hemiacetals ("furanoses") are drawn with the oxygen at the top of a pentagon. The horizontal bond at the bottom is assumed to be coming out of the plane toward you. Thus, the five-member ring is in a plane perpendicular to the page. Vertical lines are drawn on each carbon to indicate attachements above and below the plane of the 5-member ring. A furanose form of the sugar ribose is a good example:
On the left is an interactive version of the molecule; the haworth projection is in the middle, and on the right is a flat wedge/dash version. The bold bonds used in the Haworth projection are not always used, but they help emphasize the perspective. Again, bond lines with no label at the end are assumed to be to hydrogen.
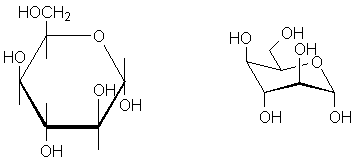
6-member rings ("pyranoses") have a slightly different but quite similar Haworth projection. A hexagon is placed so that one horizontal bond runs along the bottom. The oxygen in the ring is placed at the upper right. Usually, the hemiacetal carbon (the anomeric position) is placed at the extreme right. Again, the bond at the bottom is assumed to be coming out of the plane and vertical bonds are used to undicate substituents above and below the 6-member ring.
The left hand molecule is interactive; the middle is a Haworth projection with perspective emphasized, and the right hand molecule is a chair representation.
It is important to bear in mind that the Fischer and Haworth projections are means to keep track of stereochemistry and not necessarily an accurate depiction of the molecule.