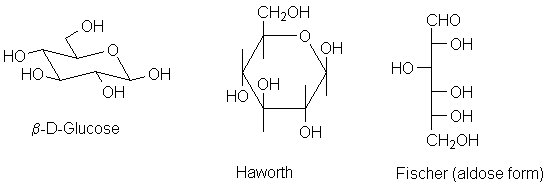
One important application of the Fischer and Haworth projections is to simplify what you have to memorize in order to talk about specific monosaccharides. If you memorize the configuration of b-D Glucose, the problem of knowing the configuration of any other hexose is to relate it to β-D-Glucose.
 |
|
| The β-anomer of D-glucose has all substituents in the equatorial position. The only other thing to remember is that the anomeric carbon is on the right hand side. Now, drawing the Haworth projection is easy; the Fischer projection can be worked out, but you should memorize it as well. | |
Now, take a look at some other important hexoses.
|
Backgrounds: Black White 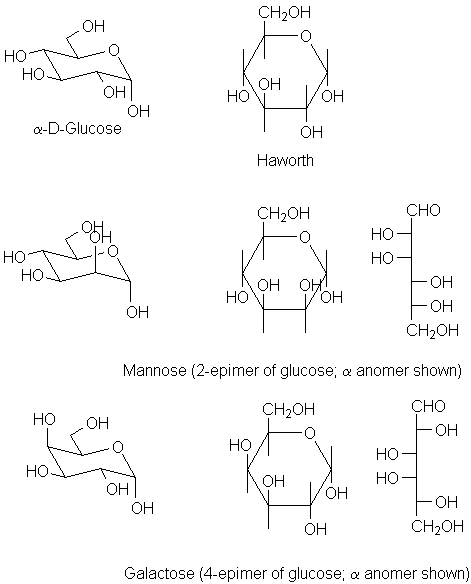 |
|
| The α-anomer of glucose differs only (obviously) at the anomeric position. | |
| Mannose has the opposite configuration only at C-2. | |
| Galactose is different from glucose in its configuration at C-4. |
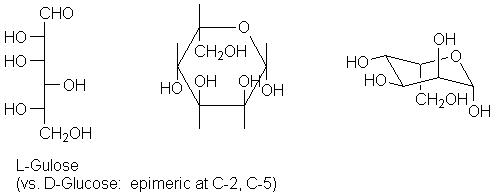
Having mastered this, now try a more difficult example. L-gulose is such a case. Gulose is one of the rarer hexoses; it differs from glucose at C-3 and C-4. However, if we consider the L-form (the enantiomer of D-gulose), it will differ from D-glucose at the 2- and 5-positions. Looking at the Fischer projection brings this out:
 |