Look at the correlation between the UV absorptions for alkenes/polyenes and the HOMO-LUMO gap:
Molecule
Calculated HOMO-LUMO gap
(B3LYP/6-31G*), eV
UV absorption
(λmax, nm)
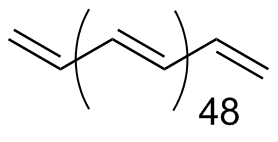
| Absorption of electromagnetic energy in the ultraviolet (UV) and visible light ranges arises from excitation of electrons from their "ground state" location to a higher-energy molecular orbital. In organic molecules, the higher energy MO is almost always the lowest-energy antibonding MO, or LUMO (Lowest Unoccupied Molecular Orbital). However, we must be conscious that in principle, any lone pair or bonding electron can be promoted, provided the impinging light photon has an energy that matches the transition. Thus we can excite lone pairs (a so-called n → π*) or π electrons (π → π*). (The * designates an antibonding MO.) Look at the correlation between the UV absorptions for alkenes/polyenes and the HOMO-LUMO gap: |
||
Molecule |
Calculated HOMO-LUMO gap(B3LYP/6-31G*), eV |
UV absorption(λmax, nm) |
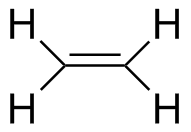
Ethylene |
7.76 | 162 |
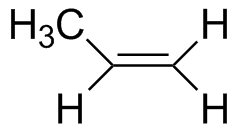
Propene |
7.55 | 170 |
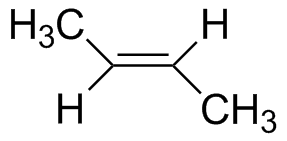
trans-2-Butene |
7.14 | 179 |
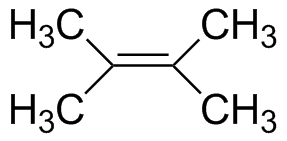
2,3-Dimethyl-2-butene |
6.55 | 196 |
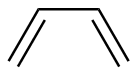
1,3-Butadiene |
5.67 | 217 |
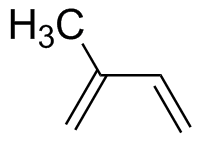
Isoprene (2-methyl-1,3-butadiene) |
5.70 | 222 |
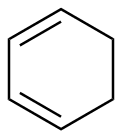
1,3-Cyclohexadiene |
5.13 | 259 |
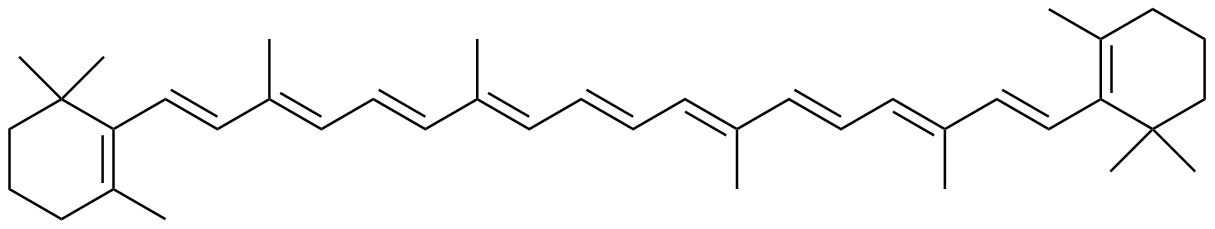
β-Carotene |
2.12 | 497 |
Polyacetylene (50-mer) |
1.49 | Reflective |