Some examples
SpectrumPlease note that many of these spectra are simulations based on data in the Spectral Database for Organic Compounds maintained by the Japanese AIST. Others are processed from raw data maintained by Pacific Lutheran University. We thank both organizations for access to this data. |
Structure & NotesFigures refer to examples in Vollhardt & Schore. |
EtClOAc.jdx |
Ethyl chloroacetate.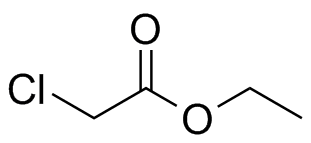 Compare to the ethyl coupling (triplet + quartet) in Ethyl Bromide, Fig. 10-21, and the compound in Fig. 10-16. |
|
iPropylIodide.jdx |
iso-Propyl Iodide.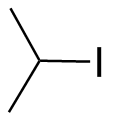 The methyl group is a doublet; the CH is a septet. Compare to Fig. 10-22. |
|
Bis_Chloroethylether_90.jdx |
bis-2-Chloroethyl ether.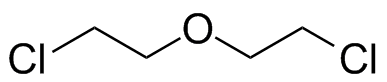 This shows the field-dependence of coupling behavior, or the appearance of it. This spectrum is at 90 MHz. (See Fig. 10-24) |
|
Bis_Chloroethylether_300 |
Now at 300 MHz. Notice how much more dispersion there is, and the degree to which we see more regular triplets. |
|
Nitropropane.jdx |
In 1-nitropropane, |
|
1Pentyne.jdx |
In 1-Pentyne, 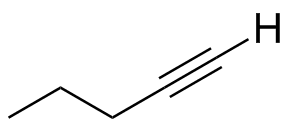 the terminal proton has a long-range (4-bond) coupling to the internal CH2 group. This in turn couples to the next CH2 group, leading to a triplet of doublets (td). the terminal proton has a long-range (4-bond) coupling to the internal CH2 group. This in turn couples to the next CH2 group, leading to a triplet of doublets (td). |
|
1Pentene.jdx |
In 1-Pentene,  the internal vinyl proton has different couplings to the terminal C-H protons (cis and trans), and a third coupling to the CH2 protons. Trace out the doublet of doublet of triplets. the internal vinyl proton has different couplings to the terminal C-H protons (cis and trans), and a third coupling to the CH2 protons. Trace out the doublet of doublet of triplets. |
Aromatic systemsAromatic couplings lead to easily recognizable patterns. Protons in an ortho relationship show large coupling (8-12 Hz, but normally about 10); protons with a meta relationship show a small coupling (2-6 Hz) and protons with a para relationship show a small or nonmeasurable coupling.Examples of ortho, meta and para-substituted rings are shown below. Identify all of the ortho and meta couplings. |
|
|
oChlorobenzoic_H.jdx |
Ortho substituted compounds often have very complex patterns because we see combinations of ortho, meta and para H-H coupling. Here is an example where one proton can be seen to be a ddd, giving 8 lines. Other are more complex and there are two proton signals that overlap. |
|
mNitrobenzaldehyde_H.jdx |
Meta disubstituted compounds usually show a singlet, two doublets and a triplet, although inequality in coupling constants can make things more complex. This example shows very little meta-coupling at all, so all of the pattern comes from the ortho H-H coupling. |
|
pNitrobenzaldehyde_H.jdx |
A para-disubstituted compound. The pair of doublets (often having some second-order lines near the baseline) is usually very clear and a real tipoff. |