Stabilities of alkenes can be measured by comparing
heats of hydrogenation.
Reaction: C=C + H2 → CH-CH
Values (in kJ/mol) taken from the NIST Webbook and reflect more current values than Wade.
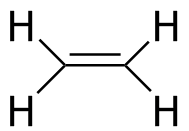
Ethylene
|
|
Monosubstituted
|
Average: -126
|
|
-136.0
|
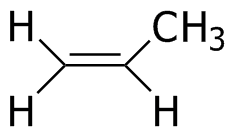
Propene
|
-123.4
|
|
|
1-Butene

|
-125.9
|
|
|
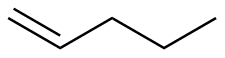
1-pentene
|
-126.0
|
|
|
1-hexene

|
-125.0
|
|
|
3-methyl-1-butene
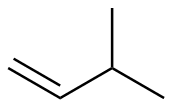
|
-126.3
|
|
|
3,3-dimethyl-1-butene
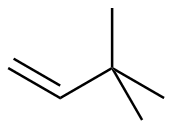
|
-125.8
|
E-disubstituted
|
Average: -115
|
Z-disubstituted
|
Average: -118
|
E-2-butene

|
-114.6
|
Z-2-butene
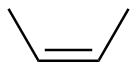
|
-118.5
|
E-2-pentene

|
-113.8
|
Z-2-pentene
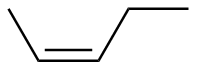
|
-117.7
|
E-2-hexene

|
-116.1
|
Z-2-hexene
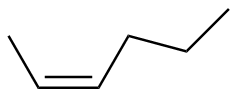
|
-119.5
|
E-3-hexene

|
-117.9
|
Z-3-hexene
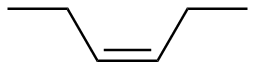
|
-121.6
|
E-4-methyl-2-butene
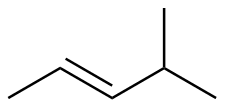
|
-114.2
|
Z-4-methyl-2-butene
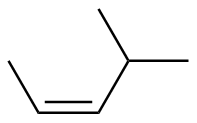
|
-116.9
|
|
|
Cyclohexene
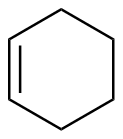
|
-118.6
|
|
|
Cyclopentene
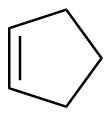
|
-112.7
|
1,1-Disubstituted
|
Average: -117
|
Trisubstituted
|
Average: -111
|
2-methylpropene
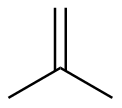
|
-117.8
|
2-methyl-2-butene
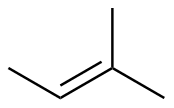
|
-111.6
|
2-methyl-1-butene
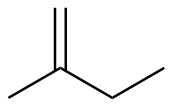
|
-118.2
|
2-methyl-2-pentene
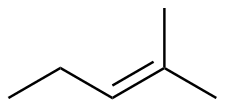
|
-111.6
|
2-methyl-1-pentene
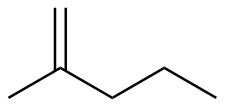
|
-116.3
|
E-3-methyl-2-pentene
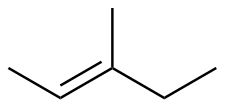
|
-110.1
|
2,3-dimethyl-1-butene
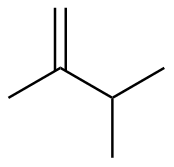
|
-116.3
|
Z-3-methyl-2-pentene
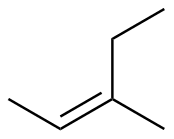
|
-110.6
|
2-ethyl-1-butene
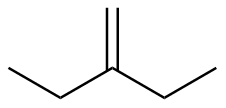
|
-115.8
|
1-methylcyclohexene
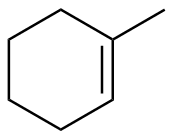
|
-111.4
|
Methylenecyclohexane
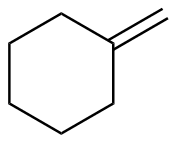
|
-119.5
|
1-methylcyclopentene
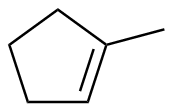
|
-100.8
|
Methylenecyclopentane
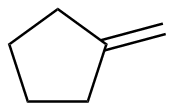
|
-115.9
|
|
|
Tetrasubstituted
|
|
|
|
2,3-dimethyl-2-butene
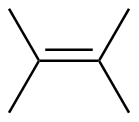
|
-110.4
|
|
|
|