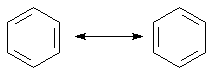
Measure bond angles
Several simple rules can help you learn how to properly use them. These are conventions that help you make sure you draw what you mean, and correctly interpret someone else's drawings.
1. The connection of two structures with the double-headed arrow signifies there is no change in nuclear position between the two structures. This is because resonance structures are separate descriptions of the same molecule. Benzene is the quintessential example.
|
|
The actual structure of benzene: |
Measure bond lengths Measure bond angles |
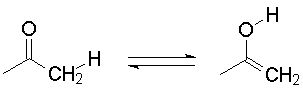
On the other hand, if you intend to show structures that are different, a proper form of notation is the double arrow, meaning an equilibrium (which may lie on either side of the arrow). An example is keto-enol tautomerism.
2. Resonance forms give us information about the degree of delocalization and, by inference, the relative stability of the molecule. The more structures that can be drawn, and the more favorable the structures, the more stable the molecule.
| Favorable | Unfavorable |
| More formal bonds | Fewer formal bonds |
| Less formal charge | More charge, and greater charge separation |
3. Placing positive charge on an electronegative element is destabilizing and characteristic of a less important contributor to overall structure than other forms.
4. Structures with an incomplete valence shell on any atom is less stable, and thus less important a contributor, than a structure with complete octets on every atom.
Rules (3) and (4) can conflict. Consider the 2-pyranyl cation:
|
|
Compare the C-O bond distance to a normal C-O single bond (1.41 Å) or C=O double bond (1.21 Å). Show the LUMO: the primary site of reaction is at C. Reset structure |
The structure on the left explains its reactivity, but the structure on the right explains why it has a short C-O bond (and why it is more stable than the cyclohexyl cation).
5. You will have gathered by now that we are devising a short-hand notation to describe the molecular orbitals arising from overlap of p-type atomic orbitals. Because the p orbitals must be parallel to lead to bonding interactions, we can state a geometric restriction:
If a formal double bond is present in any structure, all four atoms connected to the carbons in that double bond must be approximately coplanar.
An example that would violate this is seen for the 1-norbornenyl cation:
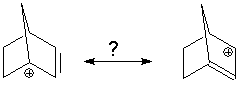 |
We might assume this is an allyl cation. For the structure on the left, careful examination of the structure shows the double bond has 4 coplanar substiituents. However, look at the substituents on the "double bond" in the structure on the right. They are definitely not coplanar so there can be no double bond between carbons 1 and 2. The right-hand structure is not a proper resonance form. |
6. The final rule is a bit obscure, but it does apply in radical chemistry. Proper resonance forms describe the same spin state; different spin states cannot be connected with the resonance arrow.
