You are here: start » activities » main
Hydrogen Wave Function Probability Densities
Highlights of the activity
- This computer visualization activity is designed to help students compute and visualize the quantum mechanical probability for finding the electron in space for the unperturbed hydrogen atom.
- Students use a Maple Worksheet or a Mathematica Notebook to visualize the probability density of the electron in the hydrogen atom and generate animations of the hydrogen orbitals that many of them have seen in chemistry.
- The whole class wrap-up discussion summarizes the solution of the Hydrogen atom with computer simulations, connecting the newly learned physics with the familiar models from introductory chemistry.
Reasons to spend class time on the activity
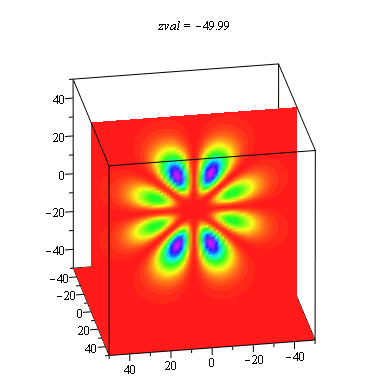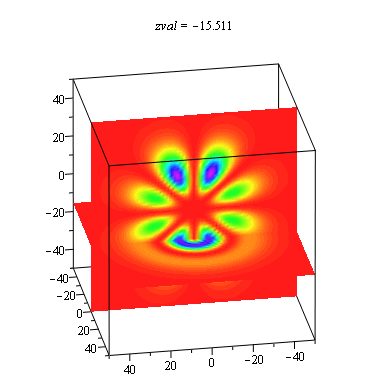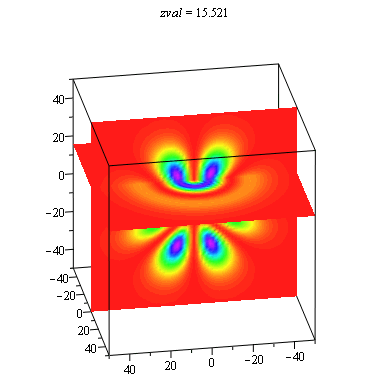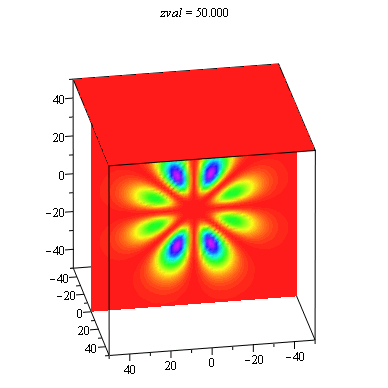
When we get to the full three-dimensional hydrogen atom problem, the choice of representation becomes especially problematic. There is not an extra dimension to use for displaying the probability density as in the ring and sphere problems. It is common to show a two-dimensional slice of the full three-dimensional distribution, and then use color or height as the probability density. We have found it useful to use the power of the computer to show all slices in an animation with each frame being a different slice. These animations allow students to explore the extent of the solutions in more depth than the traditional representations. This is especially important in order to understand that there are shell structures to many of the distributions that are not always made evident.
Reflections
Instructor's Guide
Maple Worksheet
cfhydrogenvis.mw (Maple 13)