You are here: start » courses » lecture » pplec » pplecperiodicdim
Periodic Systems in Different Dimensions (5 minutes)
- Periodic systems can take form in all three spatial dimensions:
- 3-D periodic system example: The naturally occuring photonic crystal Opal.
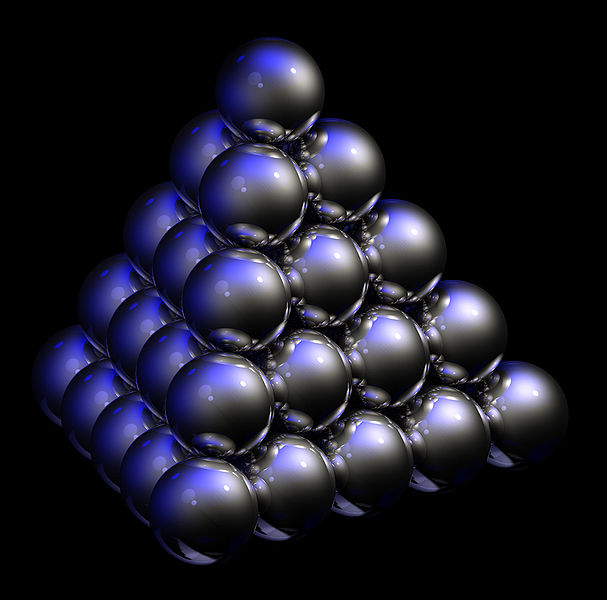
Below we can also see a cartoon interpretation of the unit cells in Opal. A 2-D periodic system can be acquired by taking only a single cross-section of unit cells in the pyramid.
Image Sources: The Wikipedia article about opal crystals.
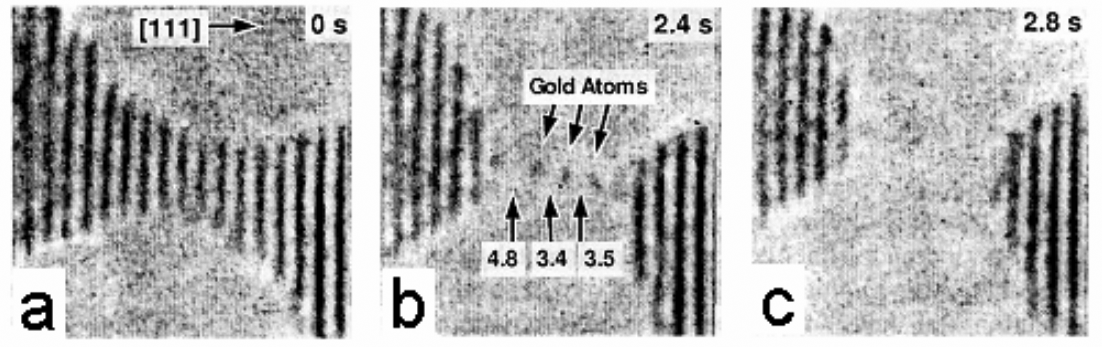
- 1-D periodic system example: a string of atoms in single-molecule conduction.
- In real physical cases, the systems always physically exist 3-Dimensionally; however, the dimensionality of the periodicity is all that concerns us now.
- Visit here for some unit cell animations. Cesium Chloride is a good example.