[_private/_private/navbar1.htm]
PROFESSOR EMERITUS(P)
B.S. Oregon State University, 1943
Ph D. California Institute of Technology, 1948
Although formally retired, Professor Hedberg maintains an active research program in
physical chemistry. His interests are in the area of molecular structure and
intramolecular dynamics.
 A knowledge or concept of the
structures of molecules is central to the interpretation of chemical results, and it is
unusual to find a scientific article in chemistry that does not draw on spatial models of
molecules that display or suggest the atoms, bonds, and bond angles. Professor Hedberg's
work is concerned with the accurate measurement of molecular structures and with the
accurate measurement of molecular structures and with the interpretation of these
measurements. The measurements are obtained by the analysis of electron-diffraction
patterns that are generated when a fine beam of electrons intersects a jet of gas.
Analysis of the patterns with the help of digital computers, often aided by additional
data from spectroscopy and quantum-mechanical calculations, leads to a spectrum of
interatomic distances such as that shown in the figure for the molecule
buckminster-fullerene. These distances allow one to construct a model of the structure and
the widths of the corresponding peaks can be interpreted to deduce properties related to
intramolecular motion. The technique of electron diffraction (ED) is the most powerful
available for studying the structures of gas molecules, and the apparatus at OSU is one of
the only few in the world.
A knowledge or concept of the
structures of molecules is central to the interpretation of chemical results, and it is
unusual to find a scientific article in chemistry that does not draw on spatial models of
molecules that display or suggest the atoms, bonds, and bond angles. Professor Hedberg's
work is concerned with the accurate measurement of molecular structures and with the
accurate measurement of molecular structures and with the interpretation of these
measurements. The measurements are obtained by the analysis of electron-diffraction
patterns that are generated when a fine beam of electrons intersects a jet of gas.
Analysis of the patterns with the help of digital computers, often aided by additional
data from spectroscopy and quantum-mechanical calculations, leads to a spectrum of
interatomic distances such as that shown in the figure for the molecule
buckminster-fullerene. These distances allow one to construct a model of the structure and
the widths of the corresponding peaks can be interpreted to deduce properties related to
intramolecular motion. The technique of electron diffraction (ED) is the most powerful
available for studying the structures of gas molecules, and the apparatus at OSU is one of
the only few in the world.
 There are several kinds of
interesting problems. One is simply the structure itself: new, unusual molecules are
sometimes put together in unusual ways that may be quickly measured by ED. Another is
vibrational force fields: the amplitudes of vibration reflected in the widths of the
distance peaks may be interpreted to give values for the force constants of the molecule.
A third is conformational analysis: many gas molecules exist as an equilibrium mixture of
rotamers, say anti and gauche forms, the proportions of which may be changed by changing
the mixture temperature. ED yields the mixture composition as well as the structures of
the components and allows one to determine the energy and entropy differences of the
components. Chemical properties that may be gotten from these measurements are strengths
of internal hydrogen bonds, shapes of torsional potentials, and the heights of barriers
hindering internal rotation. Lastly, it is possible to study the structures of materials
that are normally regarded to be involatile. Although ED is a technique applied to gases,
the vapor pressure required is very small. Sufficient sample from involatile inorganic
compounds can very often be had simply by heating them to high temperatures. There are
fascinating chemical problems connected with the structures of such materials as gases,
and Hedberg's group has constructed ovens that produce gas jets of these to enable the
preparation of diffraction patterns from them. .
There are several kinds of
interesting problems. One is simply the structure itself: new, unusual molecules are
sometimes put together in unusual ways that may be quickly measured by ED. Another is
vibrational force fields: the amplitudes of vibration reflected in the widths of the
distance peaks may be interpreted to give values for the force constants of the molecule.
A third is conformational analysis: many gas molecules exist as an equilibrium mixture of
rotamers, say anti and gauche forms, the proportions of which may be changed by changing
the mixture temperature. ED yields the mixture composition as well as the structures of
the components and allows one to determine the energy and entropy differences of the
components. Chemical properties that may be gotten from these measurements are strengths
of internal hydrogen bonds, shapes of torsional potentials, and the heights of barriers
hindering internal rotation. Lastly, it is possible to study the structures of materials
that are normally regarded to be involatile. Although ED is a technique applied to gases,
the vapor pressure required is very small. Sufficient sample from involatile inorganic
compounds can very often be had simply by heating them to high temperatures. There are
fascinating chemical problems connected with the structures of such materials as gases,
and Hedberg's group has constructed ovens that produce gas jets of these to enable the
preparation of diffraction patterns from them. .
PROFESSOR EMERITUS(P)
B.S. Oregon State University, 1943
Ph D. California Institute of Technology, 1948
Although formally retired, Professor Hedberg maintains an active research program in
physical chemistry. His interests are in the area of molecular structure and
intramolecular dynamics.
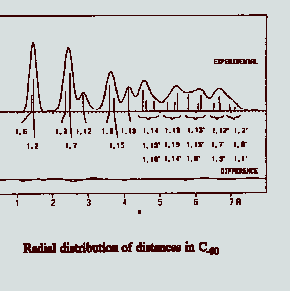 A knowledge or concept of the
structures of molecules is central to the interpretation of chemical results, and it is
unusual to find a scientific article in chemistry that does not draw on spatial models of
molecules that display or suggest the atoms, bonds, and bond angles. Professor Hedberg's
work is concerned with the accurate measurement of molecular structures and with the
accurate measurement of molecular structures and with the interpretation of these
measurements. The measurements are obtained by the analysis of electron-diffraction
patterns that are generated when a fine beam of electrons intersects a jet of gas.
Analysis of the patterns with the help of digital computers, often aided by additional
data from spectroscopy and quantum-mechanical calculations, leads to a spectrum of
interatomic distances such as that shown in the figure for the molecule
buckminster-fullerene. These distances allow one to construct a model of the structure and
the widths of the corresponding peaks can be interpreted to deduce properties related to
intramolecular motion. The technique of electron diffraction (ED) is the most powerful
available for studying the structures of gas molecules, and the apparatus at OSU is one of
the only few in the world.
A knowledge or concept of the
structures of molecules is central to the interpretation of chemical results, and it is
unusual to find a scientific article in chemistry that does not draw on spatial models of
molecules that display or suggest the atoms, bonds, and bond angles. Professor Hedberg's
work is concerned with the accurate measurement of molecular structures and with the
accurate measurement of molecular structures and with the interpretation of these
measurements. The measurements are obtained by the analysis of electron-diffraction
patterns that are generated when a fine beam of electrons intersects a jet of gas.
Analysis of the patterns with the help of digital computers, often aided by additional
data from spectroscopy and quantum-mechanical calculations, leads to a spectrum of
interatomic distances such as that shown in the figure for the molecule
buckminster-fullerene. These distances allow one to construct a model of the structure and
the widths of the corresponding peaks can be interpreted to deduce properties related to
intramolecular motion. The technique of electron diffraction (ED) is the most powerful
available for studying the structures of gas molecules, and the apparatus at OSU is one of
the only few in the world.
 There are several kinds of
interesting problems. One is simply the structure itself: new, unusual molecules are
sometimes put together in unusual ways that may be quickly measured by ED. Another is
vibrational force fields: the amplitudes of vibration reflected in the widths of the
distance peaks may be interpreted to give values for the force constants of the molecule.
A third is conformational analysis: many gas molecules exist as an equilibrium mixture of
rotamers, say anti and gauche forms, the proportions of which may be changed by changing
the mixture temperature. ED yields the mixture composition as well as the structures of
the components and allows one to determine the energy and entropy differences of the
components. Chemical properties that may be gotten from these measurements are strengths
of internal hydrogen bonds, shapes of torsional potentials, and the heights of barriers
hindering internal rotation. Lastly, it is possible to study the structures of materials
that are normally regarded to be involatile. Although ED is a technique applied to gases,
the vapor pressure required is very small. Sufficient sample from involatile inorganic
compounds can very often be had simply by heating them to high temperatures. There are
fascinating chemical problems connected with the structures of such materials as gases,
and Hedberg's group has constructed ovens that produce gas jets of these to enable the
preparation of diffraction patterns from them. .
There are several kinds of
interesting problems. One is simply the structure itself: new, unusual molecules are
sometimes put together in unusual ways that may be quickly measured by ED. Another is
vibrational force fields: the amplitudes of vibration reflected in the widths of the
distance peaks may be interpreted to give values for the force constants of the molecule.
A third is conformational analysis: many gas molecules exist as an equilibrium mixture of
rotamers, say anti and gauche forms, the proportions of which may be changed by changing
the mixture temperature. ED yields the mixture composition as well as the structures of
the components and allows one to determine the energy and entropy differences of the
components. Chemical properties that may be gotten from these measurements are strengths
of internal hydrogen bonds, shapes of torsional potentials, and the heights of barriers
hindering internal rotation. Lastly, it is possible to study the structures of materials
that are normally regarded to be involatile. Although ED is a technique applied to gases,
the vapor pressure required is very small. Sufficient sample from involatile inorganic
compounds can very often be had simply by heating them to high temperatures. There are
fascinating chemical problems connected with the structures of such materials as gases,
and Hedberg's group has constructed ovens that produce gas jets of these to enable the
preparation of diffraction patterns from them. .
 A knowledge or concept of the
structures of molecules is central to the interpretation of chemical results, and it is
unusual to find a scientific article in chemistry that does not draw on spatial models of
molecules that display or suggest the atoms, bonds, and bond angles. Professor Hedberg's
work is concerned with the accurate measurement of molecular structures and with the
accurate measurement of molecular structures and with the interpretation of these
measurements. The measurements are obtained by the analysis of electron-diffraction
patterns that are generated when a fine beam of electrons intersects a jet of gas.
Analysis of the patterns with the help of digital computers, often aided by additional
data from spectroscopy and quantum-mechanical calculations, leads to a spectrum of
interatomic distances such as that shown in the figure for the molecule
buckminster-fullerene. These distances allow one to construct a model of the structure and
the widths of the corresponding peaks can be interpreted to deduce properties related to
intramolecular motion. The technique of electron diffraction (ED) is the most powerful
available for studying the structures of gas molecules, and the apparatus at OSU is one of
the only few in the world.
A knowledge or concept of the
structures of molecules is central to the interpretation of chemical results, and it is
unusual to find a scientific article in chemistry that does not draw on spatial models of
molecules that display or suggest the atoms, bonds, and bond angles. Professor Hedberg's
work is concerned with the accurate measurement of molecular structures and with the
accurate measurement of molecular structures and with the interpretation of these
measurements. The measurements are obtained by the analysis of electron-diffraction
patterns that are generated when a fine beam of electrons intersects a jet of gas.
Analysis of the patterns with the help of digital computers, often aided by additional
data from spectroscopy and quantum-mechanical calculations, leads to a spectrum of
interatomic distances such as that shown in the figure for the molecule
buckminster-fullerene. These distances allow one to construct a model of the structure and
the widths of the corresponding peaks can be interpreted to deduce properties related to
intramolecular motion. The technique of electron diffraction (ED) is the most powerful
available for studying the structures of gas molecules, and the apparatus at OSU is one of
the only few in the world. 

