
|
PHILIP R. WATSON, PROFESSOR
B.A. Oxford University, 1974
Ph.D. University of British Columbia, 1979
Department of Chemistry, Oregon State University,
Corvallis, Oregon 97330-4003, USA
Phone : (541)-737-6740 Fax : (541)-737-2062
E-mail : watsonp@chem.orst.edu |
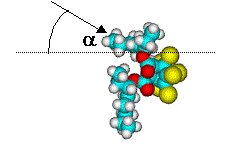
Surface orientation of a pair of head-tail molecules
Dioctyl phthalate (DOP) and bis(2-ethylhexyl)chlorendate (BEHC) are two liquids with
similar overall molecular architectures with two ethyl-substituted C6 ester
chains attached adjacent to a cyclic functionality. In the case of DOP this central
feature is an aromatic ring,
 while in
BEHC the ring is a highly chlorine-substituted non-aromatic 7-member bridgehead.
while in
BEHC the ring is a highly chlorine-substituted non-aromatic 7-member bridgehead.
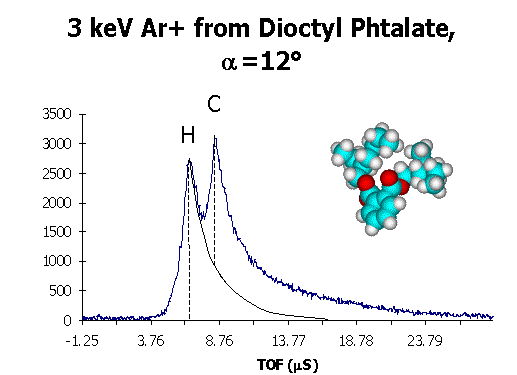
A TOF spectra for DOP taken with 3 keV Ar+ ions at an
incident angle a = 12 shows 2 major
peaks which we identify as originating from recoiling surface H and C atoms. .
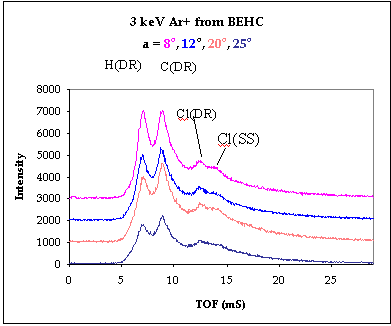
The 3 keV Ar+ BEHC spectrum shows C(DR) and H(DR)
peaks in the same positions as those from DOP. Superimposed on the long TOF tail are
other features. The peak at 12.1 Ás flight time is close to the
expected time of arrival of recoiling Cl atoms. The later feature at 13.6 Ás flight time does not correspond to any reasonable recoiling mass.
Rather we attribute this feature to a single scattering peak Cl(SS) resulting from an Ar+
ion being scattered from a Cl atom in the molecule. For 3 keV Ar+ ions single
scattering into a 45o scattering angle by collisions with H, C or O atoms is
not possible. Hence the only SS peak that we can observe is due to scattering from Cl..
The overall appearance of the DOP and BEHC spectra in the C(DR) and
H(DR) regions are very similar. The major difference is the appearance of Cl(DR) and
Cl(SS) signals on the tail of the BEHC spectrum. A difference spectrum shows this more
clearly. In the H and C regions the difference plot shows little systematic variation, but
in the Cl region the appearance of the recoil and single scattering peaks are very
apparent. The implication here is that both molecules are likely to be adopting similar
orientations at the liquid surface. The clear appearance of Cl signals in the BEHC spectra
argues that the Cl-containing ring portion of BEHC is at least partially accessible to the
incident ions. On the other hand, the large H(DR) peak in the BEHC spectra means that the
H-deficient Cl-substituted ring in BEHC cannot be predominantly at the surface with the
tails dangling below, otherwise the H signal would be very small. If DOP were exclusively
"head-up", the low H/C ratio in the head would mean that the size of the H(DR)
peak relative to the C(DR) would have to be much smaller than observed. Hence, BEHC and
DOP cannot have a "head-up" orientation.
A detailed analysis of atom ratios derived from the peak intensity ratios. The H/C
ratio for DOP orientations with one or both tails and the ring exposed produces H/C ratios
that are too low compared to the experimental value of 1.8. In order to achieve a H/C
ratio that approaches the experimental value, the DOP must be "head down",
although structures with one or both tails at the surface are essentially
indistinguishable.
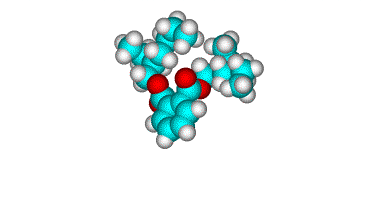
DOP - must be head down
For BEHC, orientations with most of the ring exposed have Cl/C ratios that is too high
compared to experiment. On the other hand, orientations that puts most of the ring down
has a Cl/C ratio that is too low. Orientations with 2-3 Cl atoms accessible to the ion
beam, orientations in which the ring is rotated to expose an edge and part of the
bridgehead with one or both tails in the surface, have the correct Cl/C ratio. Because the
ring in BEHC contains very little H, and the C atoms are heavily shadowed/blocked by Cl
atoms, the H/C ratio varies little with orientation and covers a range that includes the
experimental value. Hence it is difficult to say whether one or both tails are in the
surface, but it is clear that BEHC is orientated with just part of the bridgehead ring
exposed.
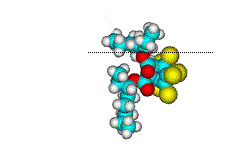
BEHC - partly head-down but some Cl exposed
For more details see :
"Orientation of molecules at a liquid surface revealed by ion scattering and
recoiling", T.J. Gannon, M. Tassotto and P. R. Watson, Chemical Physics Letters.,
300, 163-168, (1999)
Top of
page
http://www.chem.orst.edu/PersonalHomePages/Research/dopbehc.htm
Last Updated by Philip R. Watson on Monday, September 09, 2002


 while in
BEHC the ring is a highly chlorine-substituted non-aromatic 7-member bridgehead.
while in
BEHC the ring is a highly chlorine-substituted non-aromatic 7-member bridgehead.



