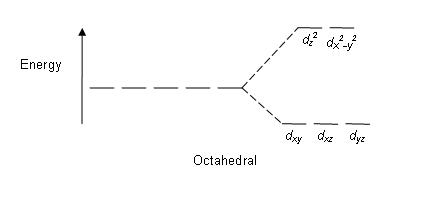
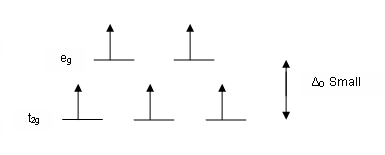
We can tell what's happening by measuring the magnetic properties of the molecule. There will be some number of unpaired electrons in each case (here the choice is 1 or 5; this will be different in each of Cr(acac)3 and Co(acac)3). Unpaired electrons make the molecule paramagnetic, and this affects its NMR properties. Unfortunately, the direct effect is that the signals for the complex (the ligand hydrogens) are broadened to the point of being undetectable. However, the unpaired electrons are both close enough to the solvent to affect its chemical shift, but still far enough away to not broaden its signal. And the specific amount of paramagnetism affects the signal coming from the solvent.
The measure of paramagnetism is the magnetic moment μeff, expressed in "Bohr magnetons", a dimensionless number that requires using all-SI units in its determination.
If n is the number of unpaired electrons,
μeff = {n(n+2)}½ (or should be).
The greater μeff, the bigger impact on the solvent chemical shift, and the whole thing is highly temperature dependent (from density considerations among other things). To determine everything we need, we have to measure:
- The change in chemical shift induced by a given molality (note that measurement: kg/m3) of solute in an NMR solvent; usually data is collected as the solution with a capillary of pure solvent in the same NMR tube.
- The temperature of the solution in the NMR probe at the time the above data is corrected.
- A correction for the intrinsic diamagnetism of the solute.
This gives us a measurement called the corrected magnetic suseptibility χMcorr which, if the units are correct, can be converted to μeff:
μeff = 797.8(TχMcorr)½
|