CH 334 Fall 2002
Worksheet #1

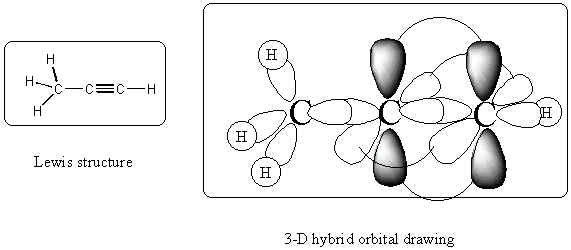
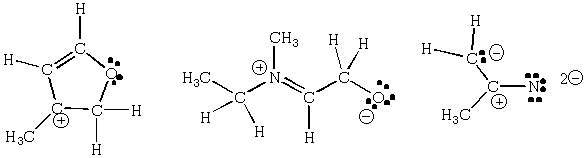
Assign hybridization to each carbon, nitrogen and oxygen in the
molecules below:
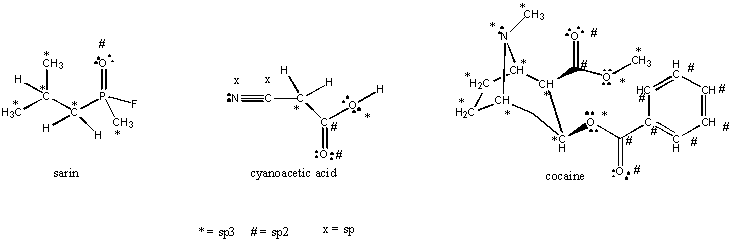
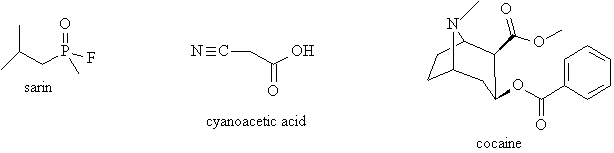
4. Sarin is a nerve gas that can be used in chemical warfare. It is an extremely active acetyl cholinesterase inhibitor which cause respiratory paralysis. The phosphorous atom in the sarin molecule seems to be breaking the octet rule. How is this possible? Phosphorous is in the third period and has additional d orbitals, so it can accommodate more than 8 electrons.