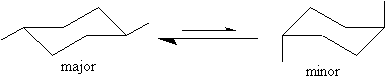
CH 334 Fall 2002
Practice Questions for Exam 1
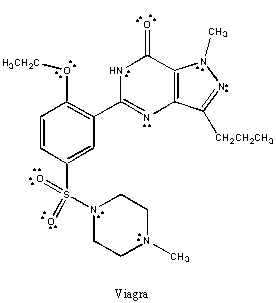
1. Sildenafil is sold under the trade name Viagra and is approved for the
treatment of impotence. Complete the following tasks on the structure of
Viagra.

2. Methyl vinyl ether has the Lewis structure shown below. In the provided space, show the 3-D hybrid orbital bonding picture of methyl vinyl ether. (10 pts)
a) Indicate hybridization of the atoms
b) Show overlap of orbitals to form bonds.
c)
 Indicate
the location of lone pairs.
Indicate
the location of lone pairs.
** note that one of the lone pairs on oxygen is conjugated to the pi bond for resonance**
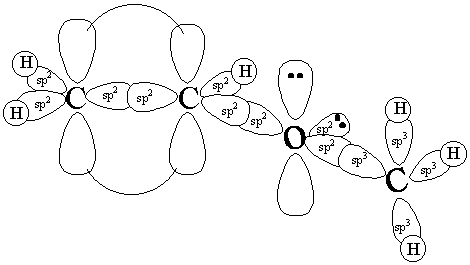
4) Rank the following in order of increasing pKa:
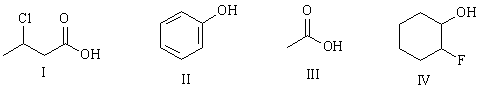
lowest pka I < III < II < IV < highest pKa
5) Draw the structural isomers of the formula C5H10 with sp3 hybridized carbons only.
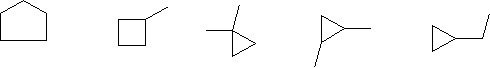
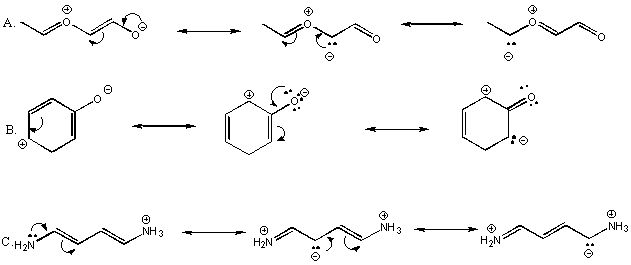
6. Show resonance forms for each of the following structures. Use the arrow formalism to indicate electron movement from one resonance form to the next.
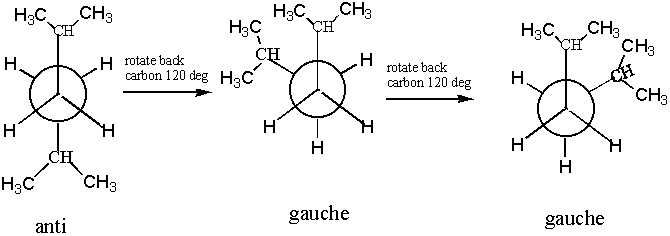
7. For the molecule 2,5-dimethylhexane, give the anti and gauche staggered Newman projections for the C(3)-C(4) bond.
8. Which one of the following IUPAC names is correct?
a) 2-ethyl-5-methylhexane c) 2,2,3-trimethylheptane
b) 2-methylcyclohexane d) 1,5-dichlorocyclohexane
9. Which of the following alkanes has the highest boiling point?
a) 2-methylpentane c) 3,3-dimethylpentane e) 2-methylhexane
b) heptane d) hexane
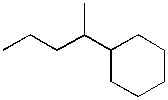
10. The following compound contains:
a) two 1°carbons, seven 2° carbons, and two 3° carbons
b) three 1° carbons, five 2° carbons, two 3° carbons, and two 4° carbons
c) two 1° carbons, five 2° carbons, two 3° carbons and two 4° carbons
d) seven 2° carbons, two 3° carbons, and two 4° carbons
11. a) Draw the two chair conformations of trans-1,4-dimethylcyclohexane.
b) Circle the structure which you expect to be the major conformation at equilibrium.