Chemistry 122 Winter 2000 Oregon State University
Exam 3 March 2, 2000 Dr. R. Nafshun/E. Chrysostom
DO NOT OPEN THIS EXAM UNTIL INSTRUCTED.
CALCULATORS ARE NOT TO BE SHARED.
Instructions: You should have with you several number two pencils, an eraser, your 3" x 5" notecard, and your University ID Card. You may use a TI-25X Solar calculator if you wish. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT. Or place the notes directly on the table at the front of the room.
Fill in the front page of the Scantron answer sheet with your last name, first name, middle initial, and student identification number. Leave the class section number and the test form number blank.
This exam consists of 16 multiple-choice questions and 4 open-ended questions. Each multiple-choice question has five points associated with it. Select the best answer by filling in the corresponding circle on the rear page of the answer sheet. If you have any questions before the exam, please ask. If you have any questions during the exam, please raise your hand to attract the attention of a proctor. The proctor will come to you. Open and start this exam when instructed. Present your ID card when submitting the exam. Place your open-ended portion of this exam in the appropriate stack. Place your 3" x 5" notecard in the appropriate stack. You may keep the multi-choice portion of this exam, so please mark the answers you selected on it.
1. Consider F2, H2, Br2, O2, and CH4. The substance with the highest boiling point is:
(a) F2.
(b) H2.
(c) Br2.
(d) O2.
(e) CH4.
2. Consider CH3CH2OCH2CH3. The intermolecular forces present in CH3CH2OCH2CH3 are:
(a) dispersion forces only.
(b) dipole-dipole forces only.
(c) dispersion forces and dipole-dipole forces only.
(d) dispersion forces, dipole-dipole forces, and hydrogen bonding.
(e) hydrogen bonding only.
3. Consider CH3CH2CH2CH2OH. The intermolecular forces present in CH3CH2CH2CH2OH are:
(a) dispersion forces only.
(b) dipole-dipole forces only.
(c) dispersion forces and dipole-dipole forces only.
(d) dispersion forces, dipole-dipole forces, and hydrogen bonding.
(e) hydrogen bonding only.
4. Consider the phase diagram below for an unknown sample at a pressure of 0.4506 atm.
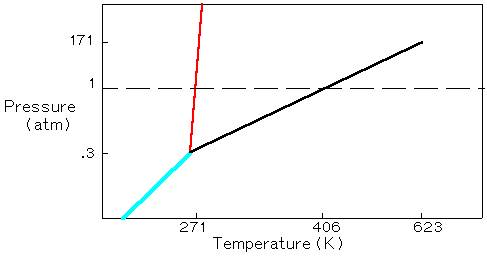
(a) At a pressure of 0.4506 atm, only the solid phase of the sample exists.
(b) At a pressure of 0.4506 atm, only the liquid phase of the sample exists.
(c) At a pressure of 0.4506 atm, only the gas phase of the sample exists.
(d) At a pressure of 0.4506 atm, only the solid and gas phases of the sample exists.
5. A student places 211.00 grams of lithium sulfate into 750.00 grams of water. The boiling point of this solution is:
(a) 102.62 °C.
(b) 103.93 °C.
(c) 105.61 °C.
(d) 106.18 °C.
(e) 108.22 °C.
6. Consider 1.0 m Na2CO3 (aq), 1.0 m KCl, 1.0 m sucrose (C12H22O11) (aq), 1.0 m AlCl3 (aq), and 1.0 m NaF (aq). The solution with the greatest boiling point is:
(a) 1.0 m Na2CO3 (aq).
(b) 1.0 m KCl.
(c) 1.0 m sucrose (aq).
(d) 1.0 m AlCl3 (aq).
(e) 1.0 m NaF (aq).
7. Consider calcium chloride, aluminum oxide, methanol, and sodium chloride. Arranged in decreasing melting point, these are:
(a) calcium chloride > methanol > aluminum oxide > sodium chloride.
(b) sodium chloride > calcium chloride > methanol > aluminum oxide.
(c) methanol > aluminum oxide > sodium chloride > calcium chloride.
(d) methanol > aluminum oxide > calcium chloride > sodium chloride.
(e) aluminum oxide > calcium chloride > sodium chloride > methanol.
8. Consider H2O at 0.50 atm, H2O at 1.0 atm, CH3CH2CH2CH3 at 0.50 atm, and CH3CH2CH2CH3 at 1.0 atm. The substance with the greatest boiling point is:
(a) H2O at 0.50 atm.
(b) H2O at 1.0 atm.
(c) CH3CH2CH2CH3 at 0.50 atm.
(d) CH3CH2CH2CH3 at 1.0 atm.
9. The phrase “rate of reaction” refers to:
(a) order of a reaction.
(b) rate of change of concentration of a reactant or product with time.
(c) integrated form of the rate equation for the reaction.
(d) rate constant for the reaction.
(e) the half-life for the reaction.
10. The balanced equation for the Haber Process (the production of ammonia) is given below
N2 + 3 H2
® 2 NH3
How is the rate of consumption of H2 related to the rate of formation of ammonia?
BE CAREFUL!!!!!
(a) - ( D[H2]/Dt) = (D[NH3]/Dt)
(b) - (1/3)( D[H2]/Dt) = (D[NH3]/Dt)
(c) - ( D[H2]/Dt) = 3(D[NH3]/Dt)
(d) - ( D[H2]/Dt) = (1/2)(D[NH3]/Dt)
(e) - ( D[H2]/Dt) = (3/2)(D[NH3]/Dt)
11. For the above reaction N2 + 3 H2 ® 2 NH3, ammonia (NH3) is forming at a rate of 0.1 Ms-1, at
what rate is N2 disappearing?
(a) 0.15 Ms-1
(b) 0.10 Ms-1
(c) 0.05 Ms-1
(d) 0.20 Ms-1
(e) 0.30 Ms-1
12. The decomposition of ozone can be written as:
2 O3 ® 3 O2 Rate = k [O3]
What is the overall order of the reaction?
(a) 0.
(b) 1.
(c) 2.
(d) 3.
(e) 4.
13. Consider the reaction A ® Products. The rate equation is known to be
Rate = k[A]m . If m = 2 and the concentration of A is doubled, the rate will
(a) stay the same.
(b) double.
(c) quadruple (x4).
(d) increase eightfold (x8).
(e) increase by a factor of sixteen (x16).
14.
Given the following kinetic data for the reaction A + 2B ® C
Experiment Initial [A], M Initial [B], M Initial Rate, Ms-1
1
0.10 0.10
5.50 x 10-6
2
0.20 0.10
2.20 x 10-5
3
0.40 0.10
8.80 x 10-5
4
0.10
0.30
1.65 x 10-5
5
0.10
0.60
3.30 x 10-5
What is the rate law for the reaction?
(a) Rate = k [A]2.
(b) Rate = k [B]2.
(c) Rate = k [A] [B].
(d) Rate = k [A]2 [B].
(e) Rate = k [A]2 [B]2.
15. The reaction SOCl2 ® SO2 + Cl2 is a first order reaction. What fraction of the original SOCl2
will remain after 3 half-lives?
(a) ½.
(b) ¼.
(c) 1/8.
(d) 1/16.
(e) ¾.
16. How does a catalyst increase the rate of a reaction?
(a) It increases the kinetic energy of the reactants.
(b) It increases the half-life of the reaction.
(c) It increases the concentration of the reactants and hence the rate.
(d) It decreases the activation energy (EA) of the reaction.
(e) All of the above.
1. [5 Points] A student places 175.0 mg of an unknown protein into 25.00 mL of water and
measures the osmotic pressure to be 17.30 torr at 25.00 °C. Determine the molar mass of the protein. [R = 0.0821 L·atm/mol·K]
2. [5 Points] Sketch a FCC unit cell and identify the equivalent number of atoms in the unit
cell.
3. [5 Points] The decomposition of H2O2 follows first order kinetics (i.e. Rate = k [H2O2]).
Given that the initial concentration of H2O2 was 0.882 M and its concentration after 225s was 0.387 M what is the rate constant (k) for the reaction?
4. [5 Points] The decomposition of N2O5 was experimentally determined to follow first order
kinetics. On the plots below clearly sketch the graphical results for such a reaction.