Chemistry 122 Winter 2000 Oregon State University
Exam 2 February 10, 2000 Dr. Richard Nafshun
DO NOT OPEN THIS EXAM UNTIL INSTRUCTED.
CALCULATORS ARE NOT TO BE SHARED.
Instructions: You should have with you several number two pencils, an eraser, your 3" x 5" notecard, and your University ID Card. You may use a TI-25X Solar calculator if you wish. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT. Or place the notes directly on the table at the front of the room.
Fill in the front page of the Scantron answer sheet with your last name, first name, middle initial, and student identification number. Leave the class section number and the test form number blank.
This exam consists of 14 multiple-choice questions and 5 open-ended questions. Each multiple-choice question has five points associated with it. Select the best answer by filling in the corresponding circle on the rear page of the answer sheet. If you have any questions before the exam, please ask. If you have any questions during the exam, please raise your hand to attract the attention of a proctor. The proctor will come to you. Open and start this exam when instructed. Present your ID card when submitting the exam. Place your open-ended portion of this exam in the appropriate stack. Place your 3" x 5" notecard in the appropriate stack. You may keep the multi-choice portion of this exam, so please mark the answers you selected on it.
1. Consider C, N, O, and F. Arranged in decreasing electronegativity, these are:
(a) C > N > O > F.
(b) F > O > N > C.
(c) N > O > F > C.
(d) N > O > C > F.
(e) C > F > O > N.
2. The F-C-F bond angle in tetrafluoromethane (CF4) is:
(a) 90°.
(b) 120°.
(c) 109.5°.
(d) Greater than 109.5°.
(e) Less than 109.5°.
3. The F-N-F bond angle in NF3 is:
(a) 90°.
(b) 120°.
(c) 109.5°.
(d) Greater than 109.5°.
(e) Less than 109.5°.
4. The O-C-O bond angle in the carbonate anion (CO32-) is:
(a) 90°.
(b) 120°.
(c) 109.5°.
(d) Greater than 109.5°.
(e) Less than 109.5°.
5. The oxygen-carbon bond order in the carbonate anion (CO32-) is:
(a) 1.00.
(b) 1.33.
(c) 1.50.
(d) 2.00.
(e) 3.00.
6. Consider CO, CO2, CO32-, and O2. Which of the following statements is correct?
(a) CO is a polar molecule.
(b) CO2 is a polar molecule.
(c) CO32- is a polar polyatomic anion.
(d) O2 is a polar molecule.
(e) CO, CO2, CO32-, and O2 are all nonpolar.
7. Consider CO2, CF4, CCl4, and NH3. Which of the following statements is correct?
(a) CO2 contains only nonpolar covalent bonds.
(b) CF4 contains only nonpolar covalent bonds.
(c) CCl4 contains only nonpolar covalent bonds.
(d) NH3 contains only nonpolar covalent bonds.
(e) CO2, CF4, CCl4, and NH3 contain only polar covalent bonds.
8. Consider C2H2, C2H4, and C2H6. Which of the following statements is correct?
(a) C2H2 has the longest carbon-carbon bond.
(b) C2H4 has the longest carbon-carbon bond.
(c) C2H6 has the longest carbon-carbon bond.
9. Consider the molecule below. The hybridization of the second carbon atom from the left is:
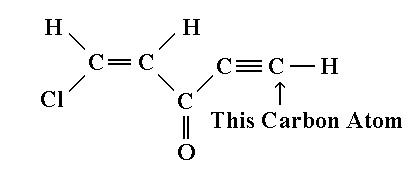
(a) s.
(b) sp.
(c) sp2.
(d) sp3.
(e) sp4.
10. Consider CH3CH2CH2CH3, CH3CHCHCH3, CH3CHC(CH3)2, and CH3CHCCl2. Which of the following statements is correct?
(a) CH3CH2CH2CH3 can exist as cis-trans isomers.
(b) CH3CHCHCH3 can exist as cis-trans isomers.
(c) CH3CHC(CH3)2 can exist as cis-trans isomers.
(d) CH3CHCCl2 can exist as cis-trans isomers.
(e) CH3CH2CH2CH3, CH3CHCHCH3, CH3CHC(CH3)2, and CH3CHCCl2 can all exist as
cis-trans isomers.
11. Consider sodium chloride, calcium chloride, aluminum oxide, and aluminum chloride. Which of the following statements is correct?
(a) Sodium chloride has the highest melting point.
(b) Calcium chloride has the highest melting point.
(c) Aluminum oxide has the highest melting point.
(d) Aluminum chloride has the highest melting point.
12. The formal charge on the nitrogen atom in ammonia is:
(a) 0.
(b) -1.
(c) +1.
(d) +3.
(e) +5.
13. Consider CH2CH2. The molecule contains:
(a) no p-bonds.
(b) one p-bond.
(c) two p-bonds.
(d) three p-bonds.
14. Molecular orbital theory predicts the F2 molecule is:
(a) diamagnetic.
(b) paramagnetic.
(c) ferromagnetic.
(d) fluoromagnetic.
(e) paulimagnetic.
YOU MUST SHOW ALL WORK TO RECEIVE CREDIT
1. [10 Points] (Part I) Sketch the molecular orbital diagram (showing all applicable atomic and
molecular orbitals with labels) for the O22+ ion. (Part II) Determine the bond order of the oxygen-oxygen bond--show your calculation. (Part III) Comment on the magnetic property of this species.
2. [3 Points] Identify and sketch a tetrahedral polar molecule indicating the molecular dipole
with a cross-based arrow.
3. [3 Points] Identify and sketch a nonpolar tetrahedral molecule.
4. [6 Points] Draw the resonance forms for the carbonate ion (CO32-).
5. [8 Points] Consider ethene (C2H4). Pictorially sketch a bonding scheme for the molecule--
labeling sigma bonds, pi bonds, atomic orbitals, and hybridized orbitals.