 Henkel
Henkel
Wash day Laundry detergent makers say they test products for effectiveness as well as environmental impact.
|
|
|||||
|
|
|
|
|||
|
|
|||||
A Fat

Fatty Acids
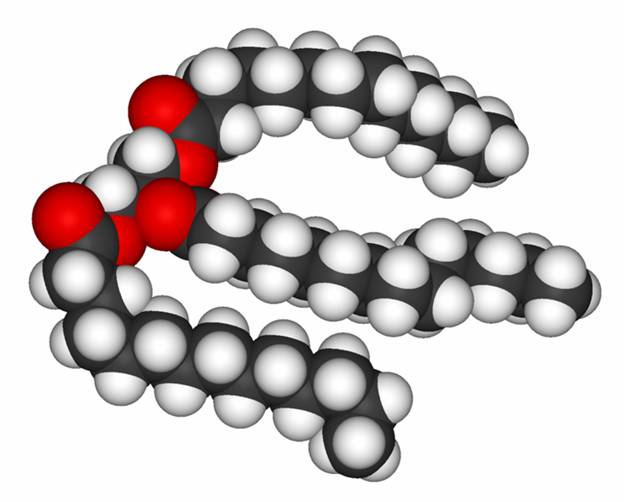
A Soap: sodium palmate
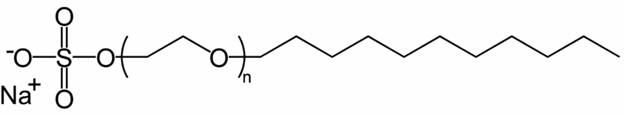
A Detergent: Sodium laureth sulfate (found in soaps, shampoos, and conditioners)
| Maximum Security Personal Care Kit | ||||
|
Super Absorbent Polymer (SAP) Link (http://www.m2polymer.com/html/superabsorbent_polymers.html)
Saran Fiber
Saran is a trade name for polyvinylidene chloride (PVDC), a substance that was inadvertently discovered in 1933 when a worker at the Dow Chemical Company was unable to fully clean a piece of glassware. Within the container was an unusually durable material that intrigued the company’s scientists when it was brought to their attention and which they soon transformed into a greenish film that they dubbed Saran.

Saran was initially utilized by the United States military, who sprayed it on fighter planes and other equipment as a protectant against the elements. Carmakers also utilized an early version of the material in upholstery to make it more resilient. Later, after scientists were able to eliminate its greenish hue and foul scent, Saran was found to be an ideal material for food packaging. By 1949, Saran Wrap was introduced by Dow as the first commercial cling wrap, and only a few years later it became a common household product used to securely wrap countless sandwiches and cover innumerable leftover items.
The molecular structure of Saran is what makes it such a useful material. Formed from the polymerization of vinylide chloride with various monomers, the molecules of Saran are bound so tightly together in such a complex design that it is extremely difficult for other molecules to penetrate it. Thus, as a film, the polyvinylidene chloride acts as a remarkable barrier against oxygen, water, and aromas. Saran is also resistant to acids, bases, and solvents, which has made it popular in the industrial sector, where it is commonly utilized in conjunction with other polymers.
The polymer structure:
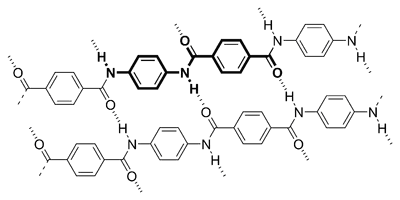
Kevlar
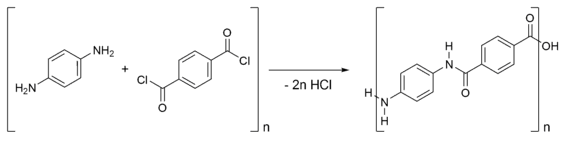
The formation of the copolymer Kevlar
By Thomas Harding Defence Correspondent
Last Updated: 2:34PM GMT 27 Feb 2009

Richard Palmer invented the D3O shock absorbing material that locks instantly into a solidified form when it is hit at high impact Photo: REUTERS
The Ministry of Defence has awarded £100,000 to a small company that has developed a special substance that hardens immediately on impact.
It is hoped that the shock-absorbing substance will soon be fitted onto the inside of soldiers' helmets reducing in half the kinetic energy of a bullet or piece of shrapnel and hopefully making them impenetrable.
The gel, called d3O locks instantly into a solidified form when it is hit at high impact.
"When moved slowly, the molecules will slip past each other, but in a high-energy impact they will snag and lock together, becoming solid," said Richard Palmer, who invented the gel. "In doing so they absorb energy."
The d3O gel has already expanded into a range of sporting goods and is found in ski gloves, shin guards, ballet shoe pointes and horse-riding equipment. The substance relies on "intelligent molecules" that "shock lock" together to absorb energy and create a solid pad. Once the pressure has gone they return to their normal flexible state.
The gel is stitched into clothing or equipment that is supple until it stiffens into a protective barrier on impact.
If the product is taken on by defence contractors it could be used to reduce the current bulky and restrictive armour used by troops in on the frontline with gel pads inserted into key protective areas.
Mr Palmer said it was the equivalent to comparing "cumbersome" RoboCop to Spiderman with the latter's protection "nimble covert and flexible".

| CH122 | 31007 | 1 | MWF | 0800-0850 | GILB 124 | Lecture | |
| CH122 | 31064 | 2 | MWF | 1000-1050 | GILB 124 | Lecture | |
| CH122 | 31069 | 3 | MWF | 1100-1150 | GILB 124 | Lecture | |
| CH122 | 31062 | 10 | T | 0800-0850 | ROG 332 | Recitation | |
| CH122 | T | 0900-1050 | GBAD 009 | Laboratory | |||
| CH122 | 31066 | 11 | T | 0900-0950 | ROG 332 | Recitation | |
| CH122 | T | 1000-1150 | GBAD 009 | Laboratory | |||
| CH122 | 34903 | 12 | T | 0900-0950 | GBAD 103 | Recitation | |
| CH122 | T | 1000-1150 | GBAD 009 | Laboratory | |||
| CH122 | 32558 | 13 | T | 1000-1050 | GBAD 103 | Recitation | |
| CH122 | T | 1100-1250 | GBAD 009 | Laboratory | |||
| CH122 | 34898 | 14 | T | 1000-1050 | ROG 332 | Recitation | |
| CH122 | T | 1100-1250 | GBAD 009 | Laboratory | |||
| CH122 | 31009 | 15 | T | 1200-1250 | GBAD 101 | Recitation | |
| CH122 | T | 1300-1450 | GBAD 009 | Laboratory | |||
| CH122 | 34895 | 16 | T | 1200-1250 | GBAD 103 | Recitation | |
| CH122 | T | 1300-1450 | GBAD 009 | Laboratory | |||
| CH122 | 31070 | 17 | T | 1300-1350 | GBAD 101 | Recitation | New room 12/17/08 |
| CH122 | T | 1400-1550 | GBAD 009 | Laboratory | |||
| CH122 | 34985 | 18 | T | 1300-1350 | GBAD 103 | Recitation | |
| CH122 | T | 1400-1550 | GBAD 009 | Laboratory | |||
| CH122 | 31065 | 19 | T | 1400-1450 | GBAD 103 | Recitation | |
| CH122 | T | 1500-1650 | GBAD 009 | Laboratory | |||
| CH122 | 34904 | 20 | T | 1400-1450 | MLM 019 | Recitation | |
| CH122 | T | 1500-1650 | GBAD 009 | Laboratory | |||
| CH122 | 31063 | 21 | W | 1100-1150 | GBAD 103 | Recitation | |
| CH122 | W | 1200-1350 | GBAD 009 | Laboratory | |||
| CH122 | 34894 | 22 | W | 1100-1150 | GILK 115 | Recitation | |
| CH122 | W | 1200-1350 | GBAD 009 | Laboratory | |||
| CH122 | 31008 | 23 | W | 1200-1250 | GBAD 101 | Recitation | |
| CH122 | W | 1300-1450 | GBAD 009 | Laboratory | |||
| CH122 | 38001 | 24 | W | 1200-1250 | GBAD 103 | Recitation | |
| CH122 | W | 1300-1450 | GBAD 009 | Laboratory | |||
| CH122 | 38002 | 25 | W | 1400-1450 | GILK 100 | Recitation | |
| CH122 | W | 1500-1650 | GBAD 009 | Laboratory | |||
| CH122 | 38003 | 26 | W | 1400-1450 | GBAD 103 | Recitation | |
| CH122 | W | 1500-1650 | GBAD 009 | Laboratory | |||
| CH122 | 31059 | 27 | R | 0800-0850 | GBAD 103 | Recitation | |
| CH122 | R | 0900-1050 | GBAD 009 | Laboratory | |||
| CH122 | 34899 | 28 | R | 0800-0850 | WNGR 201 | Recitation | |
| CH122 | R | 0900-1050 | GBAD 009 | Laboratory | |||
| CH122 | 32559 | 29 | R | 1000-1050 | GBAD 103 | Recitation | |
| CH122 | R | 1100-1250 | GBAD 009 | Laboratory | |||
| CH122 | 34902 | 30 | R | 1000-1050 | STAG 222 | Recitation | |
| CH122 | R | 1100-1250 | GBAD 009 | Laboratory | |||
| CH122 | 31067 | 31 | R | 1000-1050 | GLSN 100 | Recitation | |
| CH122 | R | 1100-1250 | GBAD 009 | Laboratory | |||
| CH122 | 34905 | 32 | R | 1000-1050 | ROG 332 | Recitation | |
| CH122 | R | 1100-1250 | GBAD 009 | Laboratory | |||
| CH122 | 31061 | 33 | R | 1200-1250 | GBAD 101 | Recitation | |
| CH122 | R | 1300-1450 | GBAD 009 | Laboratory | |||
| CH122 | 34900 | 34 | R | 1200-1250 | GBAD 103 | Recitation | |
| CH122 | R | 1300-1450 | GBAD 009 | Laboratory | |||
| CH122 | 31068 | 35 | R | 1300-1350 | GBAD 101 | Recitation | |
| CH122 | R | 1400-1550 | GBAD 009 | Laboratory | |||
| CH122 | 34906 | 36 | R | 1300-1350 | GBAD 103 | Recitation | |
| CH122 | R | 1400-1550 | GBAD 009 | Laboratory | |||
| CH122 | 31060 | 37 | R | 1400-1450 | WITH 217 | Recitation | |
| CH122 | R | 1500-1650 | GBAD 009 | Laboratory | |||
| CH122 | 34901 | 38 | R | 1400-1450 | GBAD 103 | Recitation | |
| CH122 | R | 1500-1650 | GBAD 009 | Laboratory |
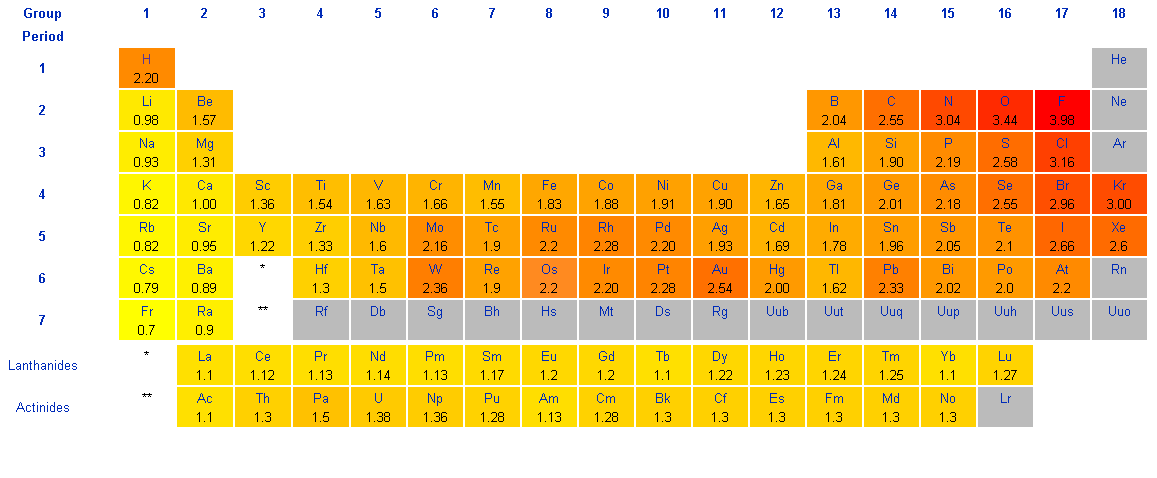
| Periodic Table with Partial Electron Configurations | ||||||||||||||||||
| H 1s1 |
He 1s2 |
|||||||||||||||||
| Li 2s1 |
Be 2s2 |
B 2s22p1 |
C 2s22p2 |
N 2s22p3 |
O 2s22p4 |
F 2s22p5 |
Ne 2s22p6 |
|||||||||||
| Na 3s1 |
Mg 3s2 |
Al 3s23p1 |
Si 3s23p2 |
P 3s23p3 |
S 3s23p4 |
Cl 3s23p5 |
Ar 3s23p6 |
|||||||||||
| K 4s1 |
Ca 4s2 |
Sc 4s23d1 |
Ti 4s23d2 |
V 4s23d3 |
Cr 4s13d5 |
Mn 4s23d5 |
Fe 4s23d6 |
Co 4s23d7 |
Ni 4s23d8 |
Cu 4s13d10 |
Zn 4s23d10 |
Ga 4s24p1 |
Ge 4s24p2 |
As 4s24p3 |
Se 4s24p4 |
Br 4s24p5 |
Kr 4s24p6 |
|
| Rb 5s1 |
Sr 5s2 |
Y 5s24d1 |
Zr 5s24d2 |
Nb 5s14d4 |
Mo 5s14d5 |
Tc 5s24d5 |
Ru 5s14d7 |
Rh 5s14d8 |
Pd 4d10 |
Ag 5s14d10 |
Cd 5s24d10 |
In 5s25p1 |
Sn 5s25p2 |
Sb 5s25p3 |
Te 5s25p4 |
I 5s25p5 |
Xe 5s25p6 |
|
| Cs 6s1 |
Ba 6s2 |
La* 6s25d1 |
Hf 6s25d2 |
Ta 6s25d3 |
W 6s25d4 |
Re 6s25d5 |
Os 6s25d6 |
Ir 6s25d7 |
Pt 6s15d9 |
Au 6s15d10 |
Hg 6s25d10 |
Tl 6s26p1 |
Pb 6s26p2 |
Bi 6s26p3 |
Po 6s26p4 |
At 6s26p5 |
Rn 6s26p6 |
|
| Fr 7s1 |
Ra 7s2 |
Ac§ 7s26d1 |
||||||||||||||||
| * | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| § | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | ||||

"I am a swimmer and the high school team that I swam for had their district meet this weekend in Eugene. My roommate and I went back to see them swim and while we were there we found this on the back of a coaches shirt. We both thought that you would like it because we remember you telling us in class the other day that chemistry will follow you everywhere...you were right! Have a good day!"

February 4, 2008

Linus Pauling is buried in the Oswego Pioneer Cemetery, only a short three minute drive from my house in Lake Oswego, Oregon, the town where he was born (then just Oswego).
While I has home this weekend, I went over a snapped a few pictures of his grave marker. He is buried by his parents, his wife and and I believe one of his sisters. I thought that you might find these pictures interesting.
February 4, 2008

"Chemistry 122 inspires art"
(We) decided to enhance our chemistry education by creating a giant periodic table on our dorm room ceiling--entirely out of recycled paper.
Attached are two pictures of the table on our ceiling. It's so big, it wouldn't even fit in one picture.
If you look closely at the pictures, the liquids are on blue paper, and the diatomic gases have their molecular formulas as their borders.
February 6, 2008
Allotropes of Carbon

Quartz (SiO2)
Silica, SiO2, phase diagram
The major phases of silica include quartz, cristobalite, tridymite, coesite, and stishovite, but there are about 50 different phases currently known; most are synthetic.

January 14, 2008
http://winter.group.shef.ac.uk/orbitron/
The quiz during Week 2 will cover atomic size trends and the sizes of parent atoms and their ions.
Molecular Geometry of Carbon in Chains:
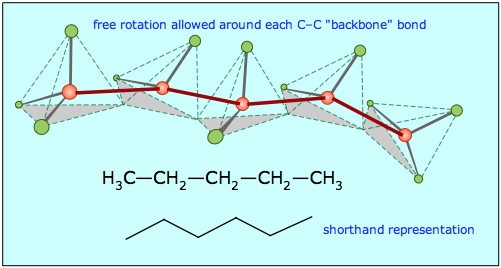

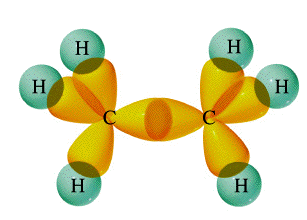
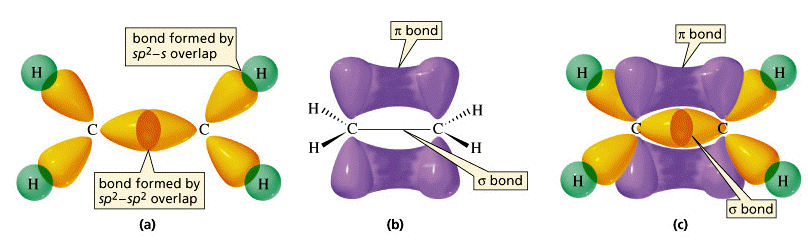
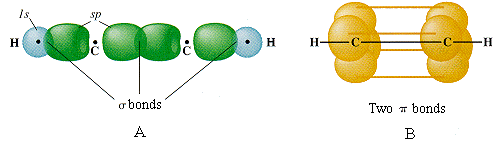


February 19, 2007
Chemical & Engineering News, January 29, 2007
ONE WOULD NOT EXPECT a Procter & Gamble laundry detergent to share advertising space in the New York Times Magazine with expensive watches and designer clothing. But this is not just any detergent; it's an environmentally friendly one.
Over the past year, the environmental impact of consumer products has gone from being a fringe issue raised mainly by activist groups to a mainstream concern confronted by P&G, Wal-Mart Stores, General Electric, and other marquee names of corporate America. The leaders of these companies no doubt have a genuine desire to improve the environmental profile of their products. But they also know green sells, and firms perceived as being kind to the environment look as good to the well-heeled readers of upscale magazines as they do to the investment community.
 Henkel
Henkel
Wash day Laundry detergent makers say they test products for effectiveness as well as environmental impact.
Because laundry detergents are bought in large volumes, mixed with water, and sent down the drain, they have been front and center in the environmental discussion. Raw materials are being scrutinized and in some cases phased out. Suppliers are being asked to come up with new, more environmentally sound ingredients to help consumer goods companies head off scrutiny or present a greener product lineup.
The P&G ad, for Tide Coldwater, is aimed at an environmentally conscious audience. It points out that if everyone in New York City washed laundry in cold water for just one day, the energy saved would be enough to light the Empire State Building for an entire month. It then suggests that Tide Coldwater, introduced in early 2005, can help accomplish this goal.
P&G is the world's largest laundry detergent company, and when it speaks, suppliers of surfactants and other raw materials listen. Thus, for Tide Coldwater, P&G's surfactant suppliers designed a hydrophobic surfactant system that solubilizes oily soils in cold water. And its enzymes suppliers developed proteases and carbohydrases that are effective on insoluble dirt residues in cold water.
 P&G
P&G
In turn, P&G and other consumer product companies must heed Wal-Mart, which has the second highest annual sales of any publicly traded company in the world. And Wal-Mart spoke loudly last fall when it announced two environmental initiatives that could profoundly affect cleaning products.
In September, at the Clinton Global Initiative in New York City, Wal-Mart launched a plan to measure its 60,000 worldwide suppliers on their ability to reduce packaging. It calculates that the plan will annually save 323,000 tons of coal and 67 million gal of diesel fuel from being burned. And it will keep some 677,000 tons of carbon dioxide out of the atmosphere.
Then in October, the company started a program to encourage the use of "preferred" ingredients in detergents and other chemical-intensive products. Wal-Mart said it would work with suppliers to substitute 20 "chemicals of concern" over the following two years. It announced the first three chemicals in October, one of which was nonylphenol ethoxylates, or NPEs, a surfactant class found in many cleaning products.
Wal-Mart's initiatives came at a time when cleaning product makers and their raw material suppliers were already reeling from a string of initiatives that are changing the way they develop their products. These include the European Union's detergents directive and its REACH chemical regulation plan, the Environmental Protection Agency's Design for the Environment program, and a state-level ban of phosphates in automatic dishwashing detergents.
But Wal-Mart's size and direct influence on the industry give its two initiatives a particular impact. And because it is a private company, Wal-Mart can immediately present its changes to suppliers and consumers without the deliberative process that government agencies must follow.
"The retailers are one step closer to the consumer and can, as Wal-Mart has proven this year, have a dramatic influence on consumer perception," says Kevin Beairsto, business manager for fabric and home care at National Starch & Chemical's Alco Chemical division. "Removal of fillers and other chemicals due to packaging and environmental concerns poses formulation challenges to detergent manufacturers and ultimately to us as ingredient suppliers."
Corporate rather than government scrutiny of chemical ingredients is a potentially game-changing development for cleaning product companies. "Corporations, such as the retailers, like to move fast," points out Ernie Rosenberg, president of the Soap & Detergent Association, the main trade group for the U.S. cleaning products industry. "A deliberative process with stakeholder participation doesn't give you a fast process."
LIKE THEM or not, lists such as Wal-Mart's 20 chemicals of concern, in Rosenberg's view, could prove to be more important arbiters of the chemical industry's product lineup than any traditional regulation. "Once it's on a list, public pressure and retailer pressure is what hits the chemical," he says. "It may get regulated down the road, but for consumer products, that may be irrelevant."
NPEs provide an instructive example of how listing, even in the absence of government action, catalyzes change. The surfactants also demonstrate how an unwanted development for one company or product is a business opportunity for other companies and products.
NPEs and other alkylphenol ethoxylates (APEs) have long been under scrutiny. According to a Sierra Club report published in November 2005, they are unlike any other cleaning agent because they break down into more toxic, less biodegradable metabolites that display estrogenic properties, such as nonylphenol itself. The APE Research Council, an industry group, contends they are safe.
Thomas Stephens, global business manager for cleaning and care at Dutch chemical maker Akzo Nobel, notes that Europe effectively banned NPEs in the 1990s for all down-the-drain applications. No legal prohibitions exist in the U.S., but EPA's Design for the Environment program recognizes companies that voluntarily phase out the manufacture and use of NPEs.
 Degussa
Degussa
Perceptive Eunjoo Kim, a chemist in Degussa's Hopewell, Va., laboratories, evaluates softness and fragrance retention benefits of ingredients offered by the company.
Not surprisingly, they have been on the decline. A recent Consumer Reports study concluded that no major U.S. brand of laundry detergent contains NPEs. Sharon J. Mitchell, vice president of R&D for P&G's global fabric care business, says her company has never formulated fabric and home care products with NPEs, "as we realized from the beginning that these chemicals could not be supported for safety at the volumes that would be required." In institutional cleaning products, where NPEs have persisted, key player JohnsonDiversey announced last summer that it would eliminate them by the end of 2006.
Yet, according to the Sierra Club report, more than 260 million lb of NPEs were consumed in the U.S. alone in 2004, and observers say they are heavily used in Asia for textile processing and other applications. Companies such as Dow Chemical, Huntsman Corp., and Rhodia say they are safe and continue to manufacture them.
"Wal-Mart's going to do what Wal-Mart's going to do," says David Parkin, Huntsman's vice president for intermediates. "We still believe NPEs are an effective cleaning agent and will provide them." At the same time, Parkin acknowledges that some companies are shunning them, and he says Huntsman is prepared to assist them with replacements such as methyl ester ethoxylates and blends of alcohol ethoxylates and ethanolamines.
In fact, while it continues to produce a chemical that concerns the likes of Wal-Mart and P&G, Huntsman last year launched a business unit charged with enhancing its practice of green chemistry that reduces the use or generation of hazardous products.
For cleaning products, Parkin says Huntsman is investigating the manufacture of propylene glycol from natural glycerin. It is using a new catalyst to combine palm-kernel-derived methyl esters and ethylene oxide to produce methyl ester ethoxylates with strong performance in cleaning applications.
"We are about providing flexibility to our customers," he says. "Although we have a good capability to develop green chemistries, there is a large market of NPE users that continue to see them as effective cleansers."
Rhodia, another NPE producer, has been researching NPE alternatives for the past five years, according to Pascal Metivier, vice president for innovation and technology at the firm's Novacare unit. "We systematically try to pursue replacements," he says. "But ultimately, the customer decides."
In the agrochemical market, where surfactants are used as emulsifiers and wetting agents, Rhodia has completely replaced NPEs with blends of other surfactants, Metivier says. Sales to detergents and other industries continue to fall, he notes, but some hang on to them because they are inexpensive and effective. "Replacing NPEs is easy. Replacing NPEs at the same cost is not," he says.
At Akzo Nobel, which also produces NPEs, Stephens says the company is developing replacements by drawing on its experience in Europe. "We have gone through the trial and error of what works and doesn't work," he says.
NPEs contain nonylphenol, a hydrophobe that is attracted to oily materials, and ethylene oxide, a water-loving appendage that keeps the molecule in solution. Although the cleaning products industry's initial direction in NPE replacement was toward linear alcohol hydrophobes, Stephens says Akzo has found branched hydrophobes to be effective without the excessive foaming often associated with linear alcohols when they are ethoxylated. A new narrow-range ethoxylation technology also lowers foaming in both linear and branched products, he adds.
While NPEs are on the wane in the U.S. mostly due to corporate initiatives, phosphate builders for automatic dishwashing (ADW) detergents are under pressure from good old-fashioned government action.
 Henkel
Henkel
Phosphates provide several functions in detergents, including hard-water neutralization, redeposition prevention, and buffering. But concerns about phosphorus-induced eutrophication in lakes and rivers led to a series of state-level bans in the 1990s that took phosphates out of most U.S. laundry detergents. ADW products were spared, though, and today many of them contain 4-8% phosphorus.
The State of Washington revoked that reprieve last June with the passage of an ADW phosphate ban intended to protect the Spokane River. It will go into effect on July 1, 2008, in three counties said to have phosphate problems and in 2010 in the rest of the state.
Whereas laundry detergent phosphates were replaced with citrates, zeolites, and other builders without major cost and performance compromises, detergent makers contend that phosphates are key to dishwasher detergent performance and that their absence will be noticed by consumers. Thomas Müller-Kirschbaum, senior vice president for R&D, technology, and supply chain with Henkel's laundry and home care business, says Henkel launched a phosphate-free ADW product in Germany about 10 years ago. "We lost market leadership and had to go back to phosphates to take the lead again," he says.
U.S. ADW detergent makers surely don't relish the prospect of coming up with a separate formula for Washington state or, worse, watching the ban spread throughout the West and across the country. Some specialty chemical companies, though, see the ban as a business opportunity whose arrival was inevitable.
"We have been telling people for some time that you can take phosphates out of ADW products and get comparable performance," says Nilesh Shah, global research director for process chemicals and biocides at Rohm and Haas.
According to Shah, effective phosphate replacement technology had been languishing on Rohm and Haas's laboratory shelves until the Washington state ban emerged. At its heart is Acusol 425N, a water-soluble acrylic polymer that, the company says, can remove food soil and keep it dispersed while controlling the deposition of carbonate scale on glassware and utensils.
At Alco, another specialty polymer supplier, Beairsto sees both pros and cons in an ADW phosphate ban. On the one hand, a number of Alco's polymers are used in current ADW formulas to prevent the filming caused by calcium phosphate scale. But Beairsto isn't surprised by the ban and, like Shah, says Alco has had zero-phosphate ADW projects under way for several years now. "We have developed several technologies that can help mitigate the negative effects of the removal of phosphates," he says.
At the specialty chemical company Croda, which acquired the surfactants maker Uniqema last September, Sales Development Manager Rob Pifer points to the Synperonic NCA series of alkoxylated copolymers, detergent boosters that allow formulators to reduce or remove the need for builders. Initially developed for the European market, the series complies with the new European Detergents Directive mandating surfactant biodegradability, he says.
Like NPEs, phosphates are effective cleaning agents unlikely to be replaced by a single ingredient. But the right combination can address ecological and toxicological concerns and still achieve the necessary functional properties, according to Tony Latella, detergents sales director in North America for BASF's performance chemicals business. He says a blend containing BASF's Trilon biodegradable chelating agent, Plurafac surfactants, and Sokalan polymers can "actually add utility and help boost product performance compared to many phosphates."
 Rohm and Haas
Rohm and Haas
New Formula The reemergence of highly concentrated detergents poses formulation challenges for ingredients suppliers.
Chemical companies are also positioning their ingredients to replace formaldehyde releasers, solvents, and other cleaning product components that may not be regulated but are seen as undesirable just the same. Shah says Rohm and Haas isothiazolone biocides are gaining as replacements for formaldehyde-releasing biocides such as dimethyl dimethylol hydantoin and 1-(3-chloroallyl)-3,5,7-triaza-1-azoniaadamantane chloride, also called quaternium 15.
Among solvents, one target is ethylene glycol butyl ether, a solvent commonly found in spray cleaners. Although EPA took EGBE off its list of Clean Air Act-regulated pollutants in 2004, it is still considered a volatile organic compound by the influential California Air Resources Board.
According to Akzo Nobel's Stephens, 1% of the company's Berol 226, a blend containing nonionic and cationic surfactants can replace the EGBE and NPE that are in many spray cleaners. One big U.S. brand made the switch in the 1990s, he says, reducing VOC emissions and increasing the product's ability to emulsify and remove gritty particulate matter. "It's one of those rare times we have shown we can do the job better for less money," he says.
ALTHOUGH BANS and regulations are real events with real repercussions, cleaning industry players insist they aren't merely being reactive. From cold-water formulas to new concentrated detergents to ingredients that come from renewable resources, fabric care companies and their raw material suppliers are trying to make sustainable products that satisfy the public's new environmental sensibility.
"We work with suppliers to ensure that all new raw materials meet stringent P&G safety criteria," says Mitchell, the P&G vice president. "P&G safety scientists are involved in this process right from the beginning, sometimes even before the actual materials are synthesized."
Above and beyond basic safety, Mitchell says, P&G often uses a "life-cycle assessment" tool to determine the water, packaging, and energy consumption associated with its products. New products that have benefited from this tool include Tide Coldwater, a single-rinse Downy fabric softener, and compact laundry detergents soon to be available in North America. The company advises Wal-Mart on the retailer's sustainability initiative, Mitchell adds, and is in its Chemical-Intensive Products Network.
P&G even backs particular ingredients to help it reach environmental goals. For years, Mitchell says, the company has worked with leading enzymes suppliers to develop enzymes that enable it to use less surfactants and other chemicals. "We are also increasing the level of naturally based surfactants in our products," she says, "using alcohols from natural oils to reduce the level of petrochemically based surfactants we use."
P&G's competitors are flying the sustainability flag as well. Henkel, a European laundry detergent leader, was one of the first companies to recognize a poorly biodegrading ingredient some 50 years ago when it removed branched alkylbenzene surfactants from its detergent formulas, according to Müller-Kirschbaum. In the past decade, he adds, Henkel has removed suspect dyestuffs from its detergents, replacing them with food-grade and other more benign dyes.
And although all detergent companies have their roots in natural soaps, Müller-Kirschbaum says Henkel has stayed more true to them than most. Today, about 35% of the surfactants it uses in laundry detergents and household products come from renewable resources, he explains. "We feel prepared to say to Wal-Mart that we are a benchmark in our industry, especially in the use of renewables."
Recent Henkel environmental initiatives in Europe include the launch last year of a new detergent, Persil Color, developed to work well at low temperatures. According to Müller-Kirschbaum, its effectiveness stems from a specially tailored stain-removal polymer, improved enzymes, and a reworked surfactant system.
Similarly, Henkel's latest ADW product, Somat 7, is formulated to clean as well at 40 oC as previous products did at 55 oC, for a 20% energy savings.
Ingredient suppliers say environment-oriented requests by customers such as P&G and Henkel have become a new part of the customer-supplier relationship. According to Rhodia's Metivier, customers have always been interested in reducing costs and gaining attributes that allow them to make new marketing claims. "What is new in the past two years is the very aggressive stance of customers on green issues," he says.
In particular, ingredient suppliers are helping customers develop detergents that perform in cold water as well as traditional products. At the 6th World Conference on Detergents in Montreux, Switzerland, in October, Per Falholt, executive vice president for R&D at the enzymes producer Novozymes, made the case for cold-water washing using detergent enzymes.
He described a life-cycle assessment of adding extra protease, lipase, and amylase enzymes to a laundry detergent while dropping the wash temperature from 40 oC to 30 oC. Wash performance improved by almost every measure, he reported, and the energy savings of making the change in Europe would be equivalent to the output of two major power plants. Falholt said Novozymes is now working on enzyme packages for washing at 20 oC.
The energy-saving companions to low-temperature detergents are highly concentrated products that answer Wal-Mart's call for less packaging. Unilever was the first big detergent maker to hit the market, with its All Small & Mighty liquid, launched in February 2006. According to Unilever, a 32-oz bottle of the new All can wash as many loads of laundry as 100 oz of traditional detergent. Unilever says its annual manufacturing and shipping savings will amount to 500 million gal of water, 26 million gal of diesel, and 150 million lb of plastic.
Concentrated detergents were rolled out in the U.S. about 15 years ago with limited success, and most of them disappeared. Patrick Cescau, Unilever's chief executive officer, told attendees at the Montreux detergents meeting that the company had been concerned the new attempt at concentrates might lead U.S. consumers to think they are getting less for their money.
To combat this impression, Unilever formed a partnership with Wal-Mart under which the retailer agreed to promote the new All to increase consumer confidence and get sales moving. "You can now find All Small & Mighty in supermarkets across the U.S.," Cescau said.
In Europe, where powdered detergents prevail, dosages have been on the decline as well, to about half the volume prevalent in the early 1990s, notes Henkel's Müller-Kirschbaum. He adds that Henkel's Dial subsidiary in the U.S. has developed a concentrated version of its Purex liquid detergent that will appear on store shelves soon.
Dave Del Guercio, director of Degussa's household care business in North America, points out that many ingredient suppliers developed concentrated detergent formulas during the market's earlier flirtation with the format. In Degussa's case, the focus is on hard-surface cleaners and fabric softeners, where the company is a leading supplier of ester-type quaternary ammonium cationic surfactants.
THE CHALLENGE of formulating concentrated cleaners is pretty straightforward, Croda's Pifer says: "How do you produce a heavy-duty product with less water in the formulation?" One of Croda's responses is Monateric 1188M, a low-toxicity additive that helps keep high-concentration formulas stable.
One result of higher surfactant concentration in liquid detergents is increased viscosity, BASF's Latella notes. Depending on the surfactant phase, formulators may be faced with unfavorable rheology, or flow properties, especially when the liquid is being poured out of the bottle. Latella says BASF's nongelling Lutensol XL and XP nonionic surfactants can help address dissolution and stability problems associated with concentrated liquids.
Rohm and Haas's Shah doesn't see formulation problems in household concentrates as much as in industrial cleaners that contain phosphates, carbonates, and other ingredients with limited solubility. "Stability becomes an issue and rheology modification can become necessary," he says. "You need to modify the liquid phase to make these cleaners work."
Do consumers know or care about the changes the cleaning products industry is making to create more environmentally improved products? Will green detergents sell?
On the one hand, detergents from the self-proclaimed "nontoxic" household products maker Seventh Generation are starting to show up in mainstream supermarkets. Farther down the aisle, though, Unilever's All Small & Mighty bottles don't mention energy and product savings. The emphasis is on convenience, and interested consumers must go to the detergent's website to learn about the environmental benefits.
Alco's Beairsto, for one, thinks consumer sentiment—or at least legislative sentiment—is moving toward more environmentally friendly products. "The real test of their conviction will come when consumers realize that the new products may either cost more or work less effectively than the products they currently use," he says. "Our challenge is to provide environmentally friendly technologies that deliver the benefits consumers have come to expect at a cost they are willing to pay."
February 20, 2007
|
nuclide |
Z(p) |
N(n) |
|
half-life |
nuclear |
representative |
range of natural |
|---|---|---|---|---|---|---|---|
|
excitation energy |
|||||||
|
8C |
6 |
2 |
8.037675(25) |
2.0(4)E-21 s [230(50) keV] |
0+ |
|
|
|
9C |
6 |
3 |
9.0310367(23) |
126.5(9) ms |
(3/2-) |
|
|
|
10C |
6 |
4 |
10.0168532(4) |
19.290(12) s |
0+ |
|
|
|
11C |
6 |
5 |
11.0114336(10) |
20.334(24) min |
3/2- |
|
|
|
12C |
6 |
6 |
12 by definition |
STABLE |
0+ |
0.9893(8) |
0.98853-0.99037 |
|
13C |
6 |
7 |
13.0033548378(10) |
STABLE |
1/2- |
0.0107(8) |
0.00963-0.01147 |
|
14C |
6 |
8 |
14.003241989(4) |
5750 y |
0+ |
|
|
|
15C |
6 |
9 |
15.0105993(9) |
2.449(5) s |
1/2+ |
|
|
|
16C |
6 |
10 |
16.014701(4) |
0.747(8) s |
0+ |
|
|
|
17C |
6 |
11 |
17.022586(19) |
193(5) ms |
(3/2+) |
|
|
|
18C |
6 |
12 |
18.02676(3) |
92(2) ms |
0+ |
|
|
|
19C |
6 |
13 |
19.03481(11) |
46.2(23) ms |
(1/2+) |
|
|
|
20C |
6 |
14 |
20.04032(26) |
16(3) ms [14(+6-5) ms] |
0+ |
|
|
|
21C |
6 |
15 |
21.04934(54)# |
<30 ns |
(1/2+)# |
|
|
|
22C |
6 |
16 |
22.05720(97)# |
6.2(13) ms [6.1(+14-12) ms] |
0+ |
|
|
Interpolation: A mathematics transitive verb to estimate the value of a mathematical function that lies between known values, often by means of a graph
Chemical & Engineering News, February 12, 2007
MECHANISTIC ENZYMOLOGY has traditionally focused on step-by-step explanations of the way enzymes do their jobs. To a large degree it still does, and there's nothing wrong with that. Such studies are enormously useful for designing new enzyme-inhibiting drugs, for example.
But some mechanistic enzymologists are adopting a broader perspective, investigating not just how enzymes work individually but also how they function in the context of the cell. An understanding of enzyme mechanisms informs the work but is no longer its main focus.
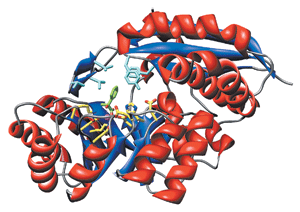 John
Gerlt & Coworkers
John
Gerlt & Coworkers
Duality Mandelate racemase (shown) and other members of the enolase superfamily have two domains: a capping domain, which includes residues (cyan) that control substrate specificity, and a barrel domain containing the enzyme's active site (primarily yellow), which includes a magnesium ion (magenta). An inhibitor (green) is bound to the active site.
Not all mechanistic enzymologists subscribe to this shift in emphasis, but the effort expended on studies of enzyme function seems to be growing. The work is motivated in part by genomic sequencing projects, which have turned up many mysterious proteins of unknown function. Their biological roles are just waiting to be discovered, like ripe fruit waiting to be picked. The move toward enzyme-function studies was a theme at the 20th Enzyme Mechanisms Conference held last month in St. Pete Beach, Fla.
"One of the current trends in the field of mechanistic enzymology is a shift in experimental focus from mechanism to cell function," said Karen N. Allen, professor of physiology and biophysics at Boston University School of Medicine. She noted "a major adjustment in the field ... as enzymologists move beyond structure-function analysis to add new tools to their arsenal." With these tools, researchers are better able to nail down the locations of different enzymes in cells, determine the substrates and protein partners with which those enzymes interact, and probe the impact of enzymes on cell metabolites.
"Sequence databases contain more unknown proteins than known proteins, and that is a real problem," said John A. Gerlt, professor of biochemistry, chemistry, and biophysics at the University of Illinois, Urbana-Champaign. It's not known, for example, whether these unknown proteins catalyze reactions, and if so, what their substrates are.
Funding agencies now recognize that determining the functions of unknown enzymes in different organisms, a pursuit known as functional genomics, warrants more encouragement and better support.
"The National Institutes of Health has put a lot of money into research on determining structures of proteins," says biochemistry professor Richard N. Armstrong of Vanderbilt University. An example is NIH's Protein Structure Initiative (PSI), a collaborative federal, academic, and industry effort to make three-dimensional protein structures more readily available. In the past, functional genomics didn't get as much funding attention as structure determination. Part of the reason is that determining the functions of proteins is more exploratory and technically much more difficult than determining their structures, "but NIH is starting to take a greater interest in this area," Armstrong said.
Determining functions of multiple proteins in cells is also a key part of systems biology, the study of relationships and interactions among various parts of biological systems or pathways. So studying cellular functions of proteins as ensembles is not a new idea. But mechanistic enzymologists bring a different perspective and their own unique expertise to the game.
At the enzyme mechanisms conference last month, Allen, Gerlt, Armstrong, and several other enzymologists described how their research programs are expanding into functional genomics. Gerlt, for example, tries to assign functions to enzymes in families whose members have similar structures and mechanisms. He discussed recent efforts by his group to ferret out the functions of enolase enzymes, which are related structurally and mechanistically and therefore constitute an enzyme superfamily.
From a structural standpoint, each member of the enolase superfamily is composed of two domains: a capping domain, which determines the enzyme's specificity for different substrates, and a barrel domain, which contains the enzyme's active site. Enolases catalyze enolization, a reaction in which a proton next to a carboxylate in the substrate is extracted by a base in the active site to generate an enolate anion. The enolase superfamily consists of some 1,700 different members, and the cellular functions of about 500 of these are unknown.
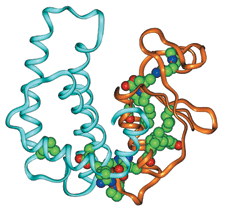 Richard Armstrong & Coworkers
Richard Armstrong & Coworkers
Family Ties Members of the glutathione transferase superfamily share a common structure as well as a set of consensus residues (shown as spheres, where green is carbon, red is oxygen, and blue is nitrogen).
To determine the cellular function of an enolase, it helps to know what its substrate is. Gerlt and coworkers try to determine this by screening enolases with libraries of potential substrates. However, "we've screened a number of unknown proteins and have failed to determine their function," he said. "We believe this is because the libraries are simply not large enough, not comprehensive enough, to be generally useful for defining function."
A better way would be to predict substrate specificity simply on the basis of the sequence and structure of specific proteins, Gerlt said. He and his coworkers, in collaboration with assistant professor of pharmaceutical chemistry Matthew Jacobson's group at the University of California, San Francisco, have tried to pursue this better path by using homology modeling (comparison with structures of known proteins) and computational docking to predict likely active sites and substrates.
In one case, they were able to confirm experimentally their theoretical prediction of an unknown enzyme's structure and substrate. Identifying this enzyme's substrate also enabled them to identify its function. "This is a very encouraging example of the ability to use homology modeling and docking, rather than libraries, to determine unknown function," Gerlt said.
Whereas Gerlt and coworkers have been determining the functions of enzymes that are similar structurally and mechanistically, Armstrong and coworkers have been doing so for members of the glutathione transferase (GST) superfamily, which are related structurally but not mechanistically.
GSTs use a common architecture to accomplish widely varying functional ends. "Similar GST structures can do many different things that are completely unrelated mechanistically," Armstrong said. And the functions of many GSTs have never been assigned. "My background in mechanistic enzymology led me to ask the questions, What are these proteins doing, and how are they doing it?" Armstrong said.
He and his coworkers use several experimental approaches to predict answers to these questions. They use gene knockouts—disabling or deleting GST genes one by one in the organism—to assess the resulting functional effects when GST enzymes encoded by those genes are missing.
They also examine the degree of expression of different GST enzymes under a variety of conditions. For example, a gene that is important for function in a specific set of circumstances, such as starvation or oxidative stress, often will be upregulated when the condition exists, and that response can be an indicator of function, Armstrong said. And they sometimes obtain crystal structures of new enzymes to look for structural clues of enzyme function.
"It turns out that GSTs are very divergent in what they do," Armstrong said. Not only do they have diverse catalytic functions, but one even turned out to regulate RNA polymerase. This function suggests that it's essentially a regulatory protein and may not actually catalyze a reaction.
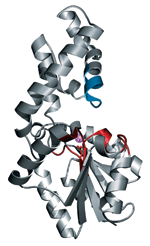 Karen Allen & Coworkers
Karen Allen & Coworkers
Division of Labor HAD enzymes have two domains: a cap domain (top half), where a loop structure (blue) controls substrate specificity, and a core domain (bottom half), which contains the active site (red) and a key magnesium ion (magenta).
Allen is also interested in finding the functions of unknown enzymes. Her group and that of her collaborator, chemistry professor Debra Dunaway-Mariano at the University of New Mexico, recently focused their efforts on the haloalkanoic acid dehalogenase (HAD) superfamily. This superfamily has about 3,800 members in prokaryotes and eukaryotes, and each organism has multiple members. Humans have about 56, for example.
Many HADs are phosphatases, enzymes that cleave phosphate groups. They all tend to have structurally related active sites that catalyze the same mechanism of phosphoryl transfer: an aspartate on the enzyme attacks and expels a substrate phosphoryl group, a phosphoenzyme intermediate forms, and a molecule of water hydrolyzes the intermediate and releases the enzyme and phosphate.
Although most HAD active sites are similar, the enzymes do differ significantly in their cap domains, where specificity-determining groups that cause the enzymes to interact with different substrates are located. "The cap domain drives the evolution of biochemical function within the HAD superfamily," Allen said.
Allen, Dunaway-Mariano, and coworkers have been investigating how variations in enzyme structure affect substrate selectivity and lead to diverse cellular functions. By using molecular modeling to dock different potential substrates into the "cage" generated by rolling a solvent molecule around the active site of different HAD enzymes, they have been able to propose, and test experimentally, functions for the enzymes.
For example, the structure of the bacterial HAD enzyme YidA had been determined previously by the New York Structural Genomics Research Consortium, part of NIH's PSI, but YidA had no known function. "We used the solvent cage derived from our analysis of the structure, in conjunction with the electrostatic and steric features of the active site, to predict potential substrates," Allen said. When they tested and validated the substrates through enzyme kinetics, the substrate with greatest activity was 5-phospho-d-arabinonate. The result suggests that YidA's cellular function involves sugar metabolism.
"It is a good example of how the marriage of structural and functional analysis yields rich results," Allen said. "I believe that in the future, enzymologists will contribute to the understanding of cell metabolism by assigning functions to those proteins whose structures are solved" as part of structural genomics efforts.
Overall, the team's functional studies of HAD superfamily members have shown how minor changes in a common structural scaffold—such as the HAD cap domain—can lead to a variety of functions. The studies also have demonstrated that genetic regulation, enzyme localization, metabolite availability, and other factors influence cell functions of different HAD enzymes, Allen noted.
John Kozarich, chairman and president of ActivX Biosciences, La Jolla, Calif., is also engaged in functional analysis of enzymes. Kozarich and coworkers have developed a technique to fish out and identify most protein kinase enzymes that are active in specific cell types.
ActivX focuses on kinases because they have widely varied cellular roles and are extraordinarily important in drug discovery. For example, approved drugs that are kinase inhibitors include the anticancer agents Gleevec and Iressa.
Mammalian cells harbor more than 500 kinases. But they're not all readily available for drug-screening purposes, their activity in different cell types is largely unknown, and knowledge of the selectivity of their interactions with different inhibitors is fragmentary.
Tag, You're It A biotin-derivatized ATP can be used to tag lysine residues in the ATP-binding sites of most active kinases. Kinases so labeled can then be isolated, identified, and quantified.
Protein kinases transfer a phosphate group from a donor compound like adenosine triphosphate (ATP) to a protein substrate. Kozarich and coworkers synthesized a biotin-containing ATP derivative that reacts with lysine residues in the ATP-binding sites of most active kinases and labels them with biotin (Biochemistry 2007, 46, 350). The labeled kinases can then be isolated, identified, and quantified.
Once identified, the selectivity of inhibitors for the various kinases can be assessed and the cellular functions of individual kinases can be studied. More than 400 different protein kinases—over 80% of the predicted human kinome—"have been identified and in most cases functionally assayed in various mammalian tissues and cell lines using this method," the researchers note in their paper.
Despite the efforts of researchers like Armstrong, Gerlt, Allen, and Kozarich, the contributions of mechanistic enzymology to functional genomics are not universally appreciated. "Biologists often do not understand the value of enzymology," said biochemistry professor Christian R. H. Raetz of Duke University Medical Center. "Deans of medical schools are likewise often unaware of the relevance of enzymology. In a sense, we have failed to communicate the value of enzymology to the broader scientific community."
Consequently, Raetz said, many scientific administrators "are spending huge amounts of money on genomics and systems biology without including people who can think at the level of chemical structure and mechanism." That may change, because chemists are now demonstrating increasingly that expertise in enzyme mechanisms can indeed provide an essential platform from which to assess the cellular functions of proteins revealed in the genomics studies of biologists.
To researchers like chemistry reader Adrian Mulholland of the University of Bristol, in England, the value of mechanistic enzymology to functional genomics and systems biology is evident. "In the U.K., we have something of a systems biology mania setting in," Mulholland said. "We are being told now that we should all be systems biologists. So it's great to come to a meeting where people are passionately interested in the fundamental chemistry of biology. This is really what we need."
ISSN 0009-2347
Copyright © 2007 American Chemical Society
February 12, 2007
Fellow faculty members,
I am forwarding an email from ASOSU President Michael Olson regarding a university student rally in support of higher education, to be held at the State Capitol on February 22. President Olson suggests several ways that you could make it possible for students to attend this rally without being penalized academically. I encourage you to seriously consider which of these suggestions make sense for the classes you are teaching that day.
The Oregon Student Association is lobbying for increases in funding that can be used to improve faculty salaries, lower student-faculty ratios, increase financial aid, and improve campus facilities. I hope you will make it easy for your students to participate in this rally, which can only help our cause and will also give university students a good lesson in civic engagement.
Thank you for your consideration,
Mike Quinn
Faculty Senate President
___
President Quinn,
I am contacting you on behalf of all OSU Students; we are planning to send over 200 students to the 2007 Higher Education Rally at the state capitol in Salem on Thursday February 22nd at 12pm. The rally is a way for the higher education community as a whole to come together in solidarity to show state legislators their constituents' support for quality, affordable post secondary education in Oregon. Statewide university students, faculty, staff, and members of the Oregon University System will attend the rally in an effort to make Oregon stronger through adequate funding of Oregon's community colleges and the Oregon University System. We have reached a breaking point in Oregon's higher education system and we must take action to demonstrate the dire need for reinvestment in Oregon.
In the past, students have made significant legislative gains as a result of their ability to turn out large numbers of constituents to the capitol for similar rallies. Statewide we would like to turn out 1200 people to the capitol and our efforts of bringing at least 200 OSU students will play a significant role in our statewide efforts. As members of the Oregon Student Association, a small group of us will also be lobbying our legislators throughout the day on our priority issues that affect access to a quality, affordable education. Our top legislative priority is funding of the system, specifically increasing faculty salaries, deferred maintenance budgets, financial aid and reducing student faculty ratios. As students, we believe that OSU's professors deserve more so that we can maintain quality at our institutions. We know that the success of this rally and our legislative efforts at making Oregon Stronger through higher education hinges on our ability to turnout large numbers of students, faculty and other supportive members of the higher education community.
In order to achieve our goal of showing Oregon State University's commitment and support for higher education we need your help! We are asking students to attend the rally on February 22. The rally is at noon so students attending will be leaving campus at 10 a.m. and arriving back around 2:00 p.m. As a professor, you have the ability to help students from OSU to make a difference in our education and fight for increased funding for faculty salaries, deferred maintenance and financial aid. Students attending the rally will have the unique opportunity to learn outside the classroom and some will be inspired to continue that learning and contribute back to their campus community. We will undoubtedly be able to send a large number of students to Salem to represent students in Oregon, provided students know their professors encourage and support their attendance at this event.
Some ways in which you might help in this effort
would be:
Informing your students of this event;
providing alternative class times for those classes scheduled to meet on
February 22nd;
providing alternative due dates for assignments or tests scheduled for that
date;
providing for make up times or assignments;
creating class assignments that incorporate interest or involvement in the
activity; and,
ensuring students have an opportunity to use "class raps" and other means to
notify their fellow students of their concerns.
This rally is equally important for all students, staff, and faculty so please help us show state legislator's OSU's commitment to making Oregon stronger. Thank you so much for your time. Please contact me with any questions or concerns.
Sincerely,
Michael F. Olson
ASOSU President
On behalf of all students at Oregon State University
Event: 2007 Higher Education Rally
Date: February 22nd, 2007
Time: 10am-2:00pm (rally at 12pm on the Capitol steps)
Meeting Location: Buses at Gill Coliseum
February 8, 2007

Metals, Nonmetals, & Semimetals (Metalloids)
Most periodic tables contain a staircase line which allows you to identify which elements are metals, nonmetals, and metalloids. Following are descriptions of each of the three types of materials.
Metals
Most elements are metals. 88 elements to the left of the staircase line are metals or metal like elements.
Physical Properties of Metals:
- Luster (shininess)
- Good conductors of heat and electricity
- High density (heavy for their size)
- High melting point
- Ductile (most metals can be drawn out into thin wires)
- Malleable (most metals can be hammered into thin sheets)
Chemical Properties of Metals:
- Easily lose electrons
- Corrode easily. Corrosion is a gradual wearing away. (Example: silver tarnishing and iron rusting)
Nonmetals
Nonmetals are found to the right of the staircase. Their characteristics are opposite those of metals.
Physical Properties of Nonmetals:
- No luster (dull appearance)
- Poor conductor of heat and electricity
- Brittle (breaks easily)
- Not ductile
- Not malleable
- Low density
- Low melting point
Chemical Properties of Nonmetals:
- Tend to gain electrons
Since metals tend to lose electrons and nonmetals tend to gain electrons, metals and nonmetals like to form compounds with each other. These compounds are called ionic compounds. When two or more nonmetals bond with each other, they form a covalent compound.
Semimetals (Metalloids)
Elements on both sides of the zigzag line have properties of both metals and nonmetals. These elements are called semimetals (metalloids).
Physical Properties of Semimetals (Metalloids):
- Solids
- Can be shiny or dull
- Ductile
- Malleable
- Conduct heat and electricity better than nonmetals but not as well as metals
February 6, 2007

Eternal embrace? Couple still hugging 5,000 years on Tue Feb 6, 1:28 PM ET Call it the eternal embrace. Archaeologists in Italy have discovered a couple buried 5,000 to 6,000 years ago, hugging each other. "It's an extraordinary case," said Elena Menotti, who led the team on their dig near the northern city of Mantova. "There has not been a double burial found in the Neolithic period, much less two people hugging -- and they really are hugging." Menotti said she believed the two, almost certainly a man and a woman although that needs to be confirmed, died young because their teeth were mostly intact and not worn down. "I must say that when we discovered it, we all became very excited. I've been doing this job for 25 years. I've done digs at Pompeii, all the famous sites," she told Reuters. "But I've never been so moved because this is the discovery of something special." A laboratory will now try to determine the couple's age at the time of death and how long they had been buried.
February 5, 2007
Human skin populated by veritable zoo of bacteria By Will Dunham Mon Feb 5, 5:06 PM ET Researchers on a safari for microbes have found that human skin is populated by a veritable menagerie of bacteria -- 182 species -- some apparently living there permanently and others just dropping by for a visit. There's no need for alarm, said microbiologist Dr. Martin Blaser of New York University School of Medicine: the bacteria have been with us for quite a while and some are helpful. In research published on Monday in the Proceedings of the National Academy of Sciences, Blaser and his colleagues took swabs from the forearms of six healthy people to study the bacterial populations in human skin -- our largest organ. "We identify about 182 species," Blaser said in an interview. "And based on those numbers, we estimate there are probably at least 250 species in the skin." "In comparison," Blaser added, "a good zoo might have 100 species or 200 species. So we already know that there are as many different species in our skin, just on the forearm, as there are in a good zoo." Bacteria are single-celled microorganisms believed to have been the first living things on Earth. While some cause disease, bacteria also reside normally in our bodies, for example in the digestive tract, performing useful chores. "Without good bacteria, the body could not survive," added Dr. Zhan Gao, a scientist in Blaser's lab involved in the study. The researchers noted that microbes in the body actually outnumber human cells 10-to-1. "Our microbes are actually, in essence, a part of our body," Blaser said. "We think that many of the normal organisms are protecting the skin. So that's why I don't think it's a great idea to keep washing all the time because we're basically washing off one of our defense layers," Blaser added. SOPHISTICATED TECHNIQUE It has long been known that bacteria reside in the skin, but Blaser and his colleagues used a sophisticated molecular technique based on DNA to conduct a rigorous census. The inhabitants proved to be more diverse than had been thought, with about 8 percent of the species previously unknown, the researchers found. Some bacteria seemed to be permanent residents of the skin, with four genera -- Staphylococcus, Streptococcus, Propionibacteria and Corynebacteria -- accounting for a bit more than half the population. Others were more transient. In each person, the population of bacteria changed over time although a core set existed for each. The volunteers included three men and three women, and the findings suggested the two sexes may differ in the bacteria they tote along. The researchers previously had studied bacteria in the stomach and esophagus. With this research, they found that the insides of the body and the skin had major differences in bacterial populations. "Microbes have been living in animals probably for a billion years. And the microbes that we have in our body are not accidental. They have evolved with us," Blaser said.
February 2, 2007
January 26, 2007
http://winter.group.shef.ac.uk/orbitron/
You will check into lab and conduct a safety orientation during Week 2--Week 3 for the Tuesday "ICE Club." You must have your legs and feet completely covered (long pants or skirt; closed toed shoes are required).
Electronegativity:
Cubic Structures:

| Isotope | Emits | Half-life |
| Uranium-238 | alpha | 4.5 billion years |
| Uranium-235 | alpha | 700 million years |
| Uranium-234 | alpha & gamma | 245,000 years |
| Plutonium-239 | alpha | 24,300 years |
| Cesium-137 | beta & gamma | 30.2 years |
| Strontium-90 | beta | 28 years |
| Cobalt-60 | beta & gamma | 5 years |
| Iodine-125 & 131 | beta & gamma | 8.1 days |
| Radionuclide | Half Life |
Radiation |
| Americium-241 | 430 years | alpha, gamma |
| Cerium-144 | 280 days | beta, gamma |
| Ruthenium-106 | 1 year | beta, gamma |
| Radionuclide | Half Life* |
|---|---|
| 3H | 4500 ± 8 d |
| 18F | 1.82951 ± 0.00034 h |
| 22Na | 950.97 ± 0.15 d |
| 24Na | 14.9512 ± 0.0032 h |
| 32P | 14.263 ± 0.003 d |
| 44Ti | 22154 ± 456 d |
| 46Sc | 83.831 ± 0.066 d |
| 51Cr | 27.7010 ± 0.0012 d |
| 54Mn | 312.028 ± 0.034 d |
| 57Co | 272.11 ± 0.26 d |
| 58Co | 70.77 ± 0.11 d |
| 59Fe | 44.5074 ± 0.0072 d |
| 60Co | 1925.20 ± 0.25 d |
| 62Cu | 9.6725 ± 0.0080 min |
| 65Zn | 244.164 ± 0.099 d |
| 67Ga | 3.26154 ± 0.00054 d |
| 75Se | 119.809 ± 0.066 d |
| 85Kr | 3935.7 ± 1.2 d |
| 85Sr | 64.8530 ± 0.0081 d |
| 88Y | 106.626 ± 0.044 d |
| 99Mo | 65.9239 ± 0.0058 h |
| 99mTc | 6.00718 ± 0.00087 h |
| 99mTc | 6.0123 ± 0.0032 h |
| 103Ru | 39.310 ± 0.044 d |
| 109Cd | 463.26 ± 0.63 d |
| 110mAg | 249.950 ± 0.024 d |
| 111In | 2.80477 ± 0.00053 d |
| 113Sn | 115.079 ± 0.080 d |
| 117mSn | 14.00 ± 0.05 d |
| 123I | 13.2235 ± 0.0019 h |
| 125I | 59.49 ± 0.13 d |
| 125Sb | 1007.56 ± 0.10 d |
| 127Xe | 36.3446 ± 0.0028 d |
| 131I | 8.0197 ± 0.0022 d |
| 131mXe | 11.934 ± 0.021 d |
| 133Ba | 3854.7 ± 2.8 d |
| 133Xe | 5.24747 ± 0.00045 d |
| 134Cs | 753.88 ± 0.15 d |
| 137Cs | 11018.3 ± 9.5 d |
| 139Ce | 137.734 ± 0.091 d |
| 140Ba | 12.7527 ± 0.0023 d |
| 140La | 40.293 ± 0.012 h |
| 141Ce | 32.510 ± 0.024 d |
| 144Ce | 284.534 ± 0.032 d |
| 152Eu | 4947.2 ± 1.1 d |
| 153Gd | 239.472 ± 0.069 d |
| 153Sm | 46.2853 ± 0.0014 h |
| 154Eu | 3145.2 ± 1.1 d |
| 155Eu | 1739.06 ± 0.45 d |
| 166Ho | 26.794 ± 0.023 h |
| 169Yb | 32.0147 ± 0.0093 d |
| 177Lu | 6.64 ± 0.01 d |
| 181W | 121.095 ± 0.064 d |
| 186Re | 89.248 ± 0.069 h |
| 188Re | 17.001 ± 0.022 h |
| 188W | 69.783 ± 0.048 d |
| 192Ir | 73.810 ± 0.019 d |
| 195Au | 186.098 ± 0.047 d |
| 198Au | 2.69517 ± 0.00021 d |
| 201Tl | 3.0456 ± 0.0015 d |
| 202Tl | 12.466 ± 0.081 d |
| 203Hg | 46.619 ± 0.027 d |
| 203Pb | 51.923 ± 0.037 h |
| 207Bi | 11523. ± 15. d |
| 228Th | 698.60 ± 0.36 d |
15-Feb-2006
Soaps:
Ethylene glycol
Ethylene glycol (monoethylene glycol (MEG), ethane-1,2-diol) is an alcohol with two -OH groups (a diol), a chemical compound widely used as an automotive antifreeze. In its pure form, it is an odorless, colorless, syrupy liquid with a sweet taste. Ethylene glycol is toxic, and its accidental ingestion should be considered a medical emergency.
12-Feb-2006
TLC Lab Lewis Structures Guide
Ethyl acetate:
Cyclohexane:
Acetone:
Ethanol (CH3CH2OH):
Carbon tetrachloride (CCl4):
Diethyl ether:
Benzene (there are two resonance forms):
Tetrahydrofuran:
o-xylene:
6-Feb-2006
http://www.stevespanglerscience.com/experiment/00000109
Please wear shoes that completely cover your feet and long pants/skirt. Wonderful weather outside, chemicals and hot solder inside.
30-Jan-2006
Signs:
In a Podiatrist's office:
"Time wounds all heels."
**************************On a Septic Tank Truck in Oregon:
Yesterday's Meals on WheelsOn another Septic Tank Truck:
"We're #1 in the #2 business."
**************************At a Proctologist's door:
"To expedite your visit please back in."
**************************On a Plumber's truck:
"We repair what your husband fixed."
**************************On another Plumber's truck:
"Don't sleep with a drip. Call your plumber.."
**************************On a Church's Billboard:
"7 days without God makes one weak."
**************************At a Tire Shop in Milwaukee:
"Invite us to your next blowout."
**************************On a Plastic Surgeon's Office door:
"Hello. Can we pick your nose?"
**************************At a Towing company:
"We don't charge an arm and a leg. We want tows."
**************************On an Electrician's truck:
"Let us remove your shorts."
**************************In a Nonsmoking Area:
"If we see smoke, we will assume you are on fire and take appropriate action."
**************************On a Maternity Room door:
"Push. Push. Push."
**************************At an Optometrist's Office:
"If you don't see what you're looking for, you've come to the right place."
**************************On a Taxidermist's window:
"We really know our stuff."
**************************On a Fence:
"Salesmen welcome! Dog food is expensive!"
**************************At a Car Dealership:
"The best way to get back on your feet - miss a car payment."
**************************Outside a Muffler Shop:
"No appointment necessary. We hear you coming."
**************************In a Veterinarian's waiting room:
"Be back in 5 minutes. Sit! Stay!"
**************************At the Electric Company:
"We would be delighted if you send in your payment.
However, if you don't, you will be."
**************************In a Restaurant window:
"Don't stand there and be hungry, Come on in and get fed up."
**************************In the front yard of a Funeral Home:
"Drive carefully. We'll wait."**************************
At a Propane Filling Station,
"Thank heaven for little grills."
**************************And don't forget the sign at a Chicago Radiator Shop:
"Best place in town to take a leak
23-Jan-2006
______________________________________________________________________________________________________________________________________
13-Jan-2006
Molecular Viewer (Chime Required)
9-Jan-2006
| s1 | s2 | |||||||||||||||||
| s1 | s2 | p1 | p2 | p3 | p4 | p5 | p6 | |||||||||||
| d1 | d2 | d3 | (d4) | d5 | d6 | d7 | d8 | (d9) | d10 | |||||||||
| f1 | (f2) | f3 | f4 | f5 | f6 | f7 | (f8) | f9 | f10 | f11 | f12 | f13 | f14 | |||||
