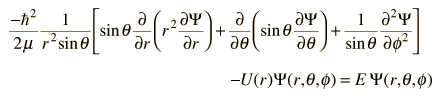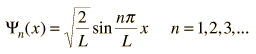Latest News
Such is life...

http://winter.group.shef.ac.uk/orbitron/

This describes what chemistry is involved in how to make fireworks
| Symbol |
Name |
Fireworks Usage |
| Al |
Aluminum |
Aluminum is used to produce silver and
white flames and sparks. It is a common component of sparklers. |
| Ba |
Barium |
Barium is used to create green colors
in fireworks, and it can also help stabilize other volatile elements. |
| Ca |
Calcium |
Calcium is used to deepen firework
colors. Calcium salts produce orange fireworks. |
| Cu |
Copper |
Copper compounds produce blue colors in
fireworks. |
| Fe |
Iron |
Iron is used to produce sparks. The
heat of the metal determines the color of the sparks. |
| Li |
Lithium |
Lithium is a metal that is used to
impart a red color to fireworks. Lithium carbonate, in particular, is a
common colorant. |
| Mg |
Magnesium |
Magnesium burns a very bright white, so
it is used to add white sparks or improve the overall brilliance of a
firework. |
| Na |
Sodium |
Sodium imparts a gold or yellow color
to fireworks, however, the color is often so bright that is frequently
masks other, less intense colors. |
| P |
Phosphorus |
Phosphorus burns spontaneously in air
and is also responsible for some glow in the dark effects. It may be a
component of a firework's fuel. |
| Sb |
Antimony |
Antimony is used to create firework
glitter effects. |
| Sr |
Strontium |
Strontium salts impart a red color to
fireworks. Strontium compounds are also important for stabilizing
fireworks mixtures. |
| Ti |
Titanium |
Titanium metal can be burned as powder
or flakes to produce silver sparks. |
| Zn |
Zinc |
Zinc is a bluish white metal that is
used to create smoke effects for fireworks and other pyrotechnic
devices. |
nuclide
symbol |
Z(p) |
N(n) |
isotopic mass (u)
|
half-life |
nuclear
spin |
representative
isotopic
composition
(mole fraction) |
range of natural
variation
(mole fraction) |
| excitation energy |
| 52Cu |
29 |
23 |
51.99718(28)# |
|
(3+)# |
|
|
| 53Cu |
29 |
24 |
52.98555(28)# |
<300 ns |
(3/2-)# |
|
|
| 54Cu |
29 |
25 |
53.97671(23)# |
<75 ns |
(3+)# |
|
|
| 55Cu |
29 |
26 |
54.96605(32)# |
40# ms [>200 ns] |
3/2-# |
|
|
| 56Cu |
29 |
27 |
55.95856(15)# |
93(3) ms |
(4+) |
|
|
| 57Cu |
29 |
28 |
56.949211(17) |
196.3(7) ms |
3/2- |
|
|
| 58Cu |
29 |
29 |
57.9445385(17) |
3.204(7) s |
1+ |
|
|
| 59Cu |
29 |
30 |
58.9394980(8) |
81.5(5) s |
3/2- |
|
|
| 60Cu |
29 |
31 |
59.9373650(18) |
23.7(4) min |
2+ |
|
|
| 61Cu |
29 |
32 |
60.9334578(11) |
3.333(5) h |
3/2- |
|
|
| 62Cu |
29 |
33 |
61.932584(4) |
9.673(8) min |
1+ |
|
|
|
63Cu |
29 |
34 |
62.9295975(6) |
STABLE |
3/2- |
0.6915(15) |
0.68983-0.69338 |
|
64Cu |
29 |
35 |
63.9297642(6) |
12.700(2) h |
1+ |
|
|
|
65Cu |
29 |
36 |
64.9277895(7) |
STABLE |
3/2- |
0.3085(15) |
0.30662-0.31017 |
| 66Cu |
29 |
37 |
65.9288688(7) |
5.120(14) min |
1+ |
|
|
| 67Cu |
29 |
38 |
66.9277303(13) |
61.83(12) h |
3/2- |
|
|
| 68Cu |
29 |
39 |
67.9296109(17) |
31.1(15) s |
1+ |
|
|
| 68mCu |
721.6(7) keV |
3.75(5) min |
(6-) |
|
|
| 69Cu |
29 |
40 |
68.9294293(15) |
2.85(15) min |
3/2- |
|
|
| 69mCu |
2741.8(10) keV |
360(30) ns |
(13/2+) |
|
|
| 70Cu |
29 |
41 |
69.9323923(17) |
44.5(2) s |
(6-) |
|
|
| 70m1Cu |
101.1(3) keV |
33(2) s |
(3-) |
|
|
| 70m2Cu |
242.6(5) keV |
6.6(2) s |
1+ |
|
|
| 71Cu |
29 |
42 |
70.9326768(16) |
19.4(14) s |
(3/2-) |
|
|
| 71mCu |
2756(10) keV |
271(13) ns |
(19/2-) |
|
|
| 72Cu |
29 |
43 |
71.9358203(15) |
6.6(1) s |
(1+) |
|
|
| 72mCu |
270(3) keV |
1.76(3) µs |
(4-) |
|
|
| 73Cu |
29 |
44 |
72.936675(4) |
4.2(3) s |
(3/2-) |
|
|
| 74Cu |
29 |
45 |
73.939875(7) |
1.594(10) s |
(1+,3+) |
|
|
| 75Cu |
29 |
46 |
74.94190(105) |
1.224(3) s |
(3/2-)# |
|
|
| 76Cu |
29 |
47 |
75.945275(7) |
641(6) ms |
(3,5) |
|
|
| 76mCu |
0(200)# keV |
1.27(30) s |
(1,3) |
|
|
| 77Cu |
29 |
48 |
76.94785(43)# |
469(8) ms |
3/2-# |
|
|
| 78Cu |
29 |
49 |
77.95196(43)# |
342(11) ms |
|
|
|
| 79Cu |
29 |
50 |
78.95456(54)# |
188(25) ms |
3/2-# |
|
|
| 80Cu |
29 |
51 |
79.96087(64)# |
100# ms [>300 ns] |
|
|
Schrödinger Equation
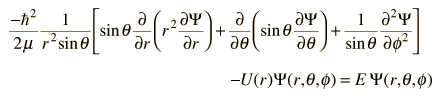
where:
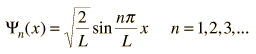
This is the form
best suited for the study of the hydrogen atom.

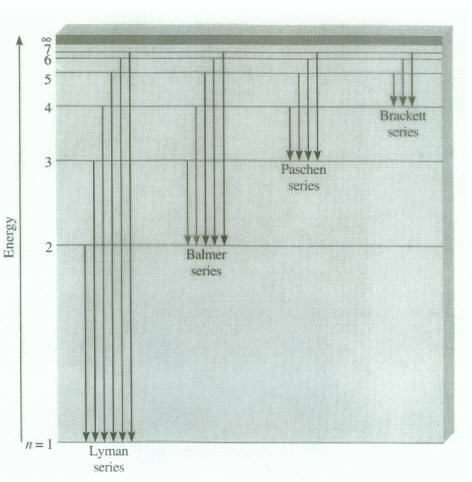




Oil on Water
Such as:
http://en.wikipedia.org/wiki/Cosco_Busan
|
Values of R |
Units
(V·P·T-1·n-1) |
|
8.314472 |
J·K-1·mol-1 |
|
0.0820574587 |
L·atm·K-1·mol-1 |
|
83.14472 |
cm3·bar·mol-1·K-1 |
|
8.20574587 × 10-5 |
m3·atm·K-1·mol-1 |
|
8.314472 |
cm3·MPa·K-1·mol-1 |
|
8.314472 |
L·kPa·K-1·mol-1 |
|
8.314472 |
m3·Pa·K-1·mol-1 |
|
62.36367 |
L·mmHg·K-1·mol-1 |
|
62.36367 |
L·Torr·K-1·mol-1 |
|
83.14472 |
L·mbar·K-1·mol-1 |
|
0.08314472 |
L·bar·K-1·mol-1 |
|
1.987 |
cal·K-1·mol-1 |
|
6.132440 |
lbf·ft·K-1·g-mol-1 |
|
10.73159 |
ft3·psi·
°R-1·lb-mol-1 |
|
0.7302413 |
ft3·atm·°R-1·lb-mol-1 |
|
998.9701 |
ft3·mmHg·K-1·lb-mol-1 |
|
8.314472 × 107 |
erg·K-1·mol-1 |
|
1716 (Air only) |
ft·lb·°R-1·slug-1 |
|
286.9 (Air only) |
N·m·kg-1·K-1 |
|
286.9 (Air only) |
J·kg-1·K-1 |
from: http://en.wikipedia.org/wiki/Gas_constant
How Many? A Dictionary of Units of Measurement © Russ
Rowlett and the University of North Carolina at Chapel Hill
The Metric System in the United States
Article I, Section 8 of the U. S. Constitution gives Congress the power to
"fix the standard of weights and measures" for the nation. The First Congress,
meeting in 1789, took up the question of weights and measures, and had the
metric system been available at that time it might have been adopted. What
actually happened is that Thomas Jefferson, who was then serving as the first
Secretary of State, submitted a report proposing a decimal-based system with a
mixture of familiar and unfamiliar names for the units.
Jefferson's system actually resembles the metric system in many ways. Its
biggest shortcoming is that Jefferson didn't hit on the idea of using prefixes
to create names for multiples of units. Consequently, his system was burdened
with a long list of names. For example, he divided his basic distance unit,
the foot (it was slightly shorter than the traditional foot) into 10 inches.
Each inch was divided into 10 lines, and each line into 10 points. For larger
distances, 10 feet equalled a decade, 100 feet was a rood, 1000 feet a
furlong, and there were 10 000 feet in a mile (making the Jeffersonian mile
about twice as long as the traditional mile). His basic volume unit was the
cubic foot, which he proposed to call a bushel (it was about 3/4 the size of a
traditional bushel). The basic weight unit was the ounce, defined so that a
bushel of water weighed 1000 ounces. (This is very similar to the metric
system, in which a liter of water weighs 1000 grams).
Congress gave this plan serious consideration, but because it lacked
independent support from other scientists it was easy to criticize.
Ultimately, Congress took no action. This left Americans with a version of the
traditional English weights and measures, including:
- distance measurements identical to those of the 1592 Act of Parliament,
- the traditional avoirdupois system of weight measurements,
- a system of measurement for dry volumes based on the "Winchester" bushel
used in England for wheat and corn since the late Middle Ages, and
- a system of measurement for liquid volumes based on the English wine
gallon of 1707.
It is remarkable that Congress never established this traditional system,
or any other system, as the mandatory system of weights and measures for the
United States. In 1832, Congress directed the Treasury Department to
standardize the measures used by customs officials at U.S. ports. The
Department adopted a report describing the traditional system, and Congress
allowed this report to stand without taking any formal action. This is the
closest the U.S. has ever come to adopting a single system of measurement.
Ironically, the U.S. missed two opportunities in 1832. Americans could have
adopted the metric system, which was then at an uncertain point in its
history; or they could have decided to align their measurements with the
British Imperial measures established by Parliament in 1824 and thus created a
possible alternative to the metric system in international commerce.
Warm Up Exercise

Calcium is a
______________________ (metal or non-metal). It is in Group Number
_________. Each calcium atom has ________ protons and
________ electrons.
Calcium can form an ionic compound (a salt) with nitrogen. The name and
formula of this compound are
______________________________
and ____________________________. This compound is composed of calcium
and nitride ions
(charged particles). How
many protons and electrons are in a calcium ion? How many protons and
electrons are in a nitride ion?
1 British thermal unit (Btu) = 1,055.05585262 joules (J)
1 calorie (cal) = 4.1868 joules (J)
1 kilowatthour (kWh) = 3.6 megajoules(MJ)
| Nutrient |
Calories per gram |
| Carbohydrate |
4 kcal |
| Protein |
4 kcal |
| Fat |
9 kcal |
| Alcohol |
7 kcal |
Table of specific heat capacities
| Substance |
Phase |
cp
J g−1 K−1 |
Cp
J mol−1 K−1 |
Cv
J mol−1 K−1 |
|
Air (Sea level, dry, 0 °C) |
gas |
1.0035 |
29.07 |
|
| Air (typical room conditionsA) |
gas |
1.012 |
29.19 |
|
|
Aluminium |
solid |
0.897 |
24.2 |
|
| Ammonia |
liquid |
4.700 |
80.08 |
|
|
Antimony |
solid |
0.207 |
25.2 |
|
| Argon |
gas |
0.5203 |
20.7862 |
12.4717 |
| Arsenic |
solid |
0.328 |
24.6 |
|
|
Beryllium |
solid |
1.82 |
16.4 |
|
| Copper |
solid |
0.385 |
24.47 |
|
| Diamond |
solid |
0.5091 |
6.115 |
|
| Ethanol |
liquid |
2.44 |
112 |
|
|
Gasoline |
liquid |
2.22 |
228 |
|
| Gold |
solid |
0.1291 |
25.42 |
|
|
Graphite |
solid |
0.710 |
8.53 |
|
| Helium |
gas |
5.1932 |
20.7862 |
12.4717 |
|
Hydrogen |
gas |
14.30 |
28.82 |
|
| Iron |
solid |
0.450 |
25.1 |
|
| Lead |
solid |
0.127 |
26.4 |
|
| Lithium |
solid |
3.58 |
24.8 |
|
|
Magnesium |
solid |
1.02 |
24.9 |
|
|
Mercury |
liquid |
0.1395 |
27.98 |
|
|
Nitrogen |
gas |
1.040 |
29.12 |
20.8 |
| Neon |
gas |
1.0301 |
20.7862 |
12.4717 |
| Oxygen |
gas |
0.918 |
29.38 |
|
|
Paraffin wax |
solid |
2.5 |
900 |
|
|
Silica (fused) |
solid |
0.703 |
42.2 |
|
| Uranium |
solid |
0.116 |
27.7 |
|
|
Water |
gas (100 °C) |
2.080 |
37.47 |
28.03 |
| liquid (25 °C) |
4.1813 |
75.327 |
74.53 |
| solid (0 °C) |
2.114 |
38.09 |
|
All
measurements are at 25 °C unless otherwise noted.
Notable minima and maxima are shown in maroon.
http://en.wikipedia.org/wiki/Specific_heat |
Oct 24, 2007 Two calculators were left in
exam rooms last week. A textbook was left in GILB 124 today.
Please see me before/after lecture to claim these items.


Oct 23, 2008 (Mole Day)

Boltzmann 3D Link

| The 9
polyatomic ions to know and write on your notecard: |
| Name
|
Formula
|
| Hydroxide
|
OH- |
| Cyanide
|
CN- |
| Nitrate
|
NO3-
|
| Acetate
|
CH3COO-
|
| Carbonate
|
CO32-
|
| Phosphate
|
PO43-
|
| Hydronium
|
H3O+
|
| Ammonium
|
NH4+ |
| Sulfate
|
SO42-
|
One day, Heisenberg was in his car speeding down the street
and he was pulled over by a police officer.
The officer came up to the window and asked, "Heisenberg, do
you have any idea how fast you were going?" Heisenberg answered, "No, but I
know exactly where I am."
---
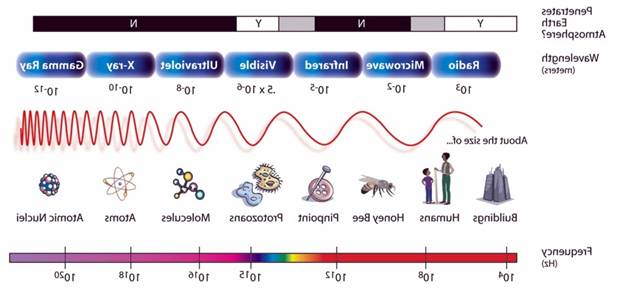
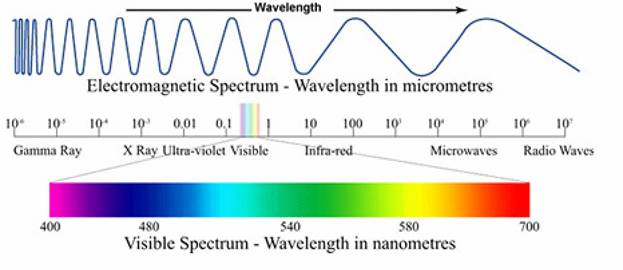
Relative Sizes
End of period
http://winter.group.shef.ac.uk/orbitron/


The heat of fusion (ΔHfusion) of water is
79.72 calories per gram or 334.5 joules per gram.
How much heat is required to change 100 g of
ice at 0 °C to water at 25 °C?
ΔHfusion
ΔHvaporization
|
Substance |
Heat of fusion
(cal/g) |
Heat of fusion
(J/g) |
|
methane: |
13.96 |
58.41 |
|
ethane: |
22.73 |
95.10 |
|
propane: |
19.11 |
79.96 |
|
methanol: |
23.70 |
99.16 |
|
ethanol: |
26.05 |
108.99 |
|
glycerol: |
47.95 |
200.62 |
|
formic acid: |
66.05 |
276.35 |
|
acetic acid: |
45.91 |
192.09 |
|
acetone: |
23.42 |
97.99 |
|
benzene: |
30.45 |
127.40 |
|
myristic acid: |
47.49 |
198.70 |
|
palmitic acid: |
39.18 |
163.93 |
|
stearic acid: |
47.54 |
198.91 |
|
|
Element |
Heat of
vaporization (kJ/mol) |
|
Methanol |
37.4 |
|
Ammonia |
23.35 |
|
Water |
40.65 |
|
Methane |
8.19 |
|
Phosphine |
14.6 |
|
Propane |
356 kJ/kg |
|
Butane |
362 kJ/kg |
|
|
|
Substance |
Phase |
cp
J g-1 K-1 |
Cp
J mol-1 K-1 |
|
Air (Sea
level,dry,0°C) |
gas |
1.0035 |
29.07 |
|
Air (typical room
conditions) |
gas |
1.012 |
29.19 |
|
Aluminum |
solid |
0.897 |
24.2 |
|
Ammonia |
liquid |
4.700 |
80.08 |
|
Argon |
gas |
0.5203 |
20.7862 |
|
Beryllium |
solid |
1.82 |
16.4 |
|
Copper |
solid |
0.385 |
24.47 |
|
Diamond |
solid |
0.5091 |
6.115 |
|
Ethanol |
liquid |
2.44 |
112 |
|
Gold |
solid |
0.1291 |
25.42 |
|
Graphite |
solid |
0.710 |
8.53 |
|
Helium |
gas |
5.1932 |
20.7862 |
|
Hydrogen |
gas |
14.30 |
28.82 |
|
Iron |
solid |
0.450 |
25.1 |
|
Lithium |
solid |
3.58 |
24.8 |
|
Mercury |
liquid |
0.1395 |
27.98 |
|
Nitrogen |
gas |
1.040 |
29.12 |
|
Neon |
gas |
1.0301 |
20.7862 |
|
Oxygen |
gas |
0.918 |
29.38 |
|
Silica (fused) |
solid |
0.703 |
42.2 |
|
Uranium |
solid |
0.116 |
27.7 |
|
Water |
gas (100°C) |
2.080 |
37.47 |
|
liquid (25°C) |
4.1813 |
75.327 |
|
solid (0°C) |
2.114 |
38.09 |
|
All measurements
are at 25 °C unless otherwise noted. |
|
Usually of interest to
builders and solar designers
|
Substance |
Phase |
cp
J g-1 K-1 |
|
Asphalt |
solid |
0.92 |
|
Brick |
solid |
0.84 |
|
Concrete |
solid |
0.88 |
|
Glass, crown |
solid |
0.67 |
|
Glass, flint |
solid |
0.503 |
|
Glass, pyrex |
solid |
0.753 |
|
Granite |
solid |
0.790 |
|
Gypsum |
solid |
1.09 |
|
Marble, mica |
solid |
0.880 |
|
Sand |
solid |
0.835 |
|
Soil |
solid |
0.80 |
|
Wood |
solid |
0.42 |
|
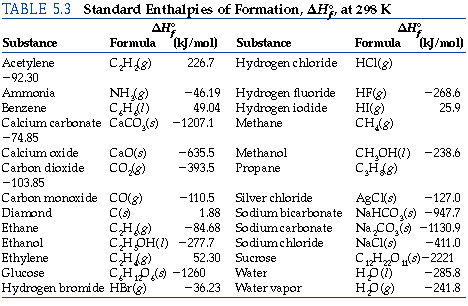
Examples: Inorganic compounds (at 25 °C)
- (State: g - gaseous; l - liquid; s - solid; aq = aqueous)
| Compound
|
DHf (kJ/mol) |
Compound
|
DHf (kJ/mol) |
| AgBr(s) |
-99.5 |
C2H2(g) |
+226.7 |
| AgCl(s) |
-127.0 |
C2H4(g) |
+52.3 |
| AgI(s) |
-62.4 |
C2H6(g) |
-84.7 |
| Ag2O(s) |
-30.6 |
C3H8(g) |
-103.8 |
| Ag2S(s) |
-31.8 |
n-C4H10(g) |
-124.7 |
| Al2O3(s) |
-1669.8 |
n-C5H12(l) |
-173.1 |
| BaCl2(s) |
-860.1 |
C2H5OH(l) |
-277.6 |
| BaCO3(s) |
-1218.8 |
CoO(s) |
-239.3 |
| BaO(s) |
-558.1 |
Cr2O3(s) |
-1128.4 |
| BaSO4(s) |
-1465.2 |
CuO(s) |
-155.2 |
| CaCl2(s) |
-795.0 |
Cu2O(s) |
-166.7 |
| CaCO3 |
-1207.0 |
CuS(s) |
-48.5 |
| CaO(s) |
-635.5 |
CuSO4(s) |
-769.9 |
| Ca(OH)2(s) |
-986.6 |
Fe2O3(s) |
-822.2 |
| CaSO4(s) |
-1432.7 |
Fe3O4(s) |
-1120.9 |
| CCl4(l) |
-139.5 |
HBr(g) |
-36.2 |
| CH4(g) |
-74.8 |
HCl(g) |
-92.3 |
| CHCl3(l) |
-131.8 |
HF(g) |
-268.6 |
| CH3OH(l) |
-238.6 |
HI(g) |
+25.9 |
| CO(g) |
-110.5 |
HNO3(l) |
-173.2 |
| CO2(g) |
-393.5 |
H2O(g) |
-241.8 |
| H2O(l) |
-285.8 |
NH4Cl(s) |
-315.4 |
| H2O2(l) |
-187.6 |
NH4NO3(s) |
-365.1 |
| H2S(g) |
-20.1 |
NO(g) |
+90.4 |
| H2SO4(l) |
-811.3 |
NO2(g) |
+33.9 |
| HgO(s) |
-90.7 |
NiO(s) |
-244.3 |
| HgS(s) |
-58.2 |
PbBr2(s) |
-277.0 |
| KBr(s) |
-392.2 |
PbCl2(s) |
-359.2 |
| KCl(s) |
-435.9 |
PbO(s) |
-217.9 |
| KClO3(s) |
-391.4 |
PbO2(s) |
-276.6 |
| KF(s) |
-562.6 |
Pb3O4(s) |
-734.7 |
| MgCl2(s) |
-641.8 |
PCl3(g) |
-306.4 |
| MgCO3(s) |
-1113 |
PCl5(g) |
-398.9 |
| MgO(s) |
-601.8 |
SiO2(s) |
-859.4 |
| Mg(OH)2(s) |
-924.7 |
SnCl2(s) |
-349.8 |
| MgSO4(s) |
-1278.2 |
SnCl4(l) |
-545.2 |
| MnO(s) |
-384.9 |
SnO(s) |
-286.2 |
| MnO2(s) |
-519.7 |
SnO2(s) |
-580.7 |
| NaCl(s) |
-411.0 |
SO2(g) |
-296.1 |
| NaF(s) |
-569.0 |
So3(g) |
-395.2 |
| NaOH(s) |
-426.7 |
ZnO(s) |
-348.0 |
| NH3(g) |
-46.2 |
ZnS(s) |
-202.9 |
Salicylic acid

C7H6O3


Scientists Announce Creation of Atomic
Element, the Heaviest Yet
By Rick Weiss
Washington Post Staff Writer
Tuesday, October 17, 2006; A03
Scientists in California and Russia announced yesterday that
they have created the heaviest atomic element ever made, adding a new item
to the universal menu of matter known as the periodic table and revealing
fresh secrets about the nature of atoms, the fundamental units of physical
stuff.
The new, radioactive element, which has not yet been
formally named but is being referred to variously as ununoctium (Latin for
"one-one-eight"), eka-radon (beneath radon on the periodic table) or simply
element 118, did not linger long.
Indeed, as with most "super-heavy" elements -- which are not
known to exist in nature but have been synthesized by slamming smaller atoms
together -- the three atoms of ununoctium created in the latest experiments
came and went in a literal flash.
But during their brief tenures of about nine ten-thousandths
of a second each in a laboratory on Russia's Volga River, those three atoms
revealed much about the laws that govern the behavior of matter, scientists
said.
And while practical applications for such fleeting phenomena
are difficult to envision, experts said they were confident some would
appear -- especially if researchers can leverage the findings to make even
larger atomic constructs that might have lifetimes of minutes, months or
longer.
"One never knows what the application of the things you find
may be," said Darleane Hoffman, a professor of chemistry at the University
of California at Berkeley, tossing out the example of plutonium-239, the key
fissile ingredient in atomic bombs, first created in 1941.
Physicists cautioned that the finding must be considered
provisional for now. That is true of all experiments that have yet to be
independently replicated, but especially so for the finding of element 118,
whose discovery was first reported by a Berkeley team in 1999 and then
retracted two years later when it became clear that the results were
fraudulent.
The last new element to be confirmed was No. 111,
roentgenium, discovered in 1994.
But scientists involved in the new find -- and others who
reviewed the report, published in the October issue of the journal Physical
Review C -- said they were virtually certain that what they saw in that
millimoment was indeed a microhunk of ununoctium.
"I would say we're very confident," said team member Nancy
Stoyer of the Lawrence Livermore National Laboratory in Livermore, Calif.,
estimating that the odds of the result being false were less than 1 in
10,000.
The team was led by Dawn Shaughnessy of Livermore and Yuri
Oganessian of the Joint Institute for Nuclear Research in Dubna, Russia.
Every naturally occurring thing in the universe is made from
a modest celestial palette of 92 elements, from hydrogen to uranium. Each
element has an atomic number (from 1 to 92) representing the number of
positively charged protons in that atom's core, or nucleus. Many variants,
or isotopes, of each element also exist through the addition of varying
numbers of uncharged neutrons to those nuclei.
For decades, scientists have been making new elements,
heavier than any found in nature, in part to help them understand the basic
forces that hold atoms together and keep them apart. They also want to know
the biggest element that can be made. Theory predicts a finite limit.
The technique involves spraying a target made of one kind of
atom with atomic buckshot of another kind and hoping that a few of the
incoming nuclei will hit a few of the target atoms with enough force to
overcome their mutually repulsive positive charges and merge into one giant
nucleus, at least briefly. To accomplish that requires a combination of
ultra-precise engineering and outlandish brute force.
In the latest experiments, which took more than 3,000 hours,
the researchers fired about 40 billion billion atoms of calcium-48 -- a
heavy, neutron-laden version of calcium -- at a target of californium-249, a
highly radioactive synthetic element. Special sensors detected a total of
three atoms of ununoctium flying off as a result of those painstaking
efforts -- one in an experiment in 2002, and two in early 2005.
Each quickly threw off a pair of protons and a pair of
neutrons to make element 116, then did so again to make element 114, and
again to make element 112, which then split in two.
It is that trail of "daughters" that allows scientists to
infer that a "mother" atom was there in the first place. But that kind of
proof is tricky, said Walter Loveland, a chemistry professor at Oregon State
University, because the super-heavy daughters are so poorly understood
themselves.
Still, Loveland said he found the results "impressive and
internally very self-consistent" and "a tremendous intellectual
achievement."
One major question left unanswered by the experiment is
whether there are super-heavy elements yet to be made that will be far more
stable -- a predicted phenomenon that scientists have called "an island of
stability."
An isotope of element 114, discovered by Livermore
scientists, showed preliminary but now uncertain evidence of unusual
longevity, on the order of 20 seconds. Some had predicted that ununoctium
might stick around long enough for researchers to do some chemistry on it.
The new work, while undermining that idea, offers new information that will
help theoreticians revamp their predictions, which can then be tested by
experimentalists.
"We're nibbling away at the shores of the island of
stability," said Livermore's Ken Moody.
Metric Prefixes:
| yotta- (Y-) |
1024 |
1 septillion |
| zetta- (Z-) |
1021 |
1 sextillion |
| exa- (E-) |
1018 |
1 quintillion |
| peta- (P-) |
1015 |
1 quadrillion |
| tera- (T-) |
1012 |
1 trillion |
| giga- (G-) |
109 |
1 billion |
| mega- (M-) |
106 |
1 million |
| kilo- (k-) |
103 |
1 thousand |
| hecto- (h-) |
102 |
1 hundred |
| deka- (da-)** |
10 |
1 ten |
| deci- (d-) |
10-1 |
1 tenth |
| centi- (c-) |
10-2 |
1 hundredth |
| milli- (m-) |
10-3 |
1 thousandth |
| micro- (µ-) |
10-6 |
1 millionth |
| nano- (n-) |
10-9 |
1 billionth |
| pico- (p-) |
10-12 |
1 trillionth |
| femto- (f-) |
10-15 |
1 quadrillionth |
| atto- (a-) |
10-18 |
1 quintillionth |
| zepto- (z-) |
10-21 |
1 sextillionth |
| yocto- (y-) |
10-24 |
1 septillionth |
and...
1 ångström (Å) = 10–10 meters = 0.1 nm = 100
pm For an example of lengths in this unit, the average diameter of an atom,
calculated from its empirical radius, ranges from approximately 0.5 Å for
hydrogen (the smallest element) to 3.8 Å for uranium (the largest naturally
occurring element on earth).
Need more?
http://en.wikipedia.org/wiki/Conversion_of_units
Polyatomic Ions:
| The 9 polyatomic ions to know and write on your notecard: |
| Name |
Formula |
| Hydroxide |
OH- |
| Cyanide |
CN- |
| Nitrate |
NO3- |
| Acetate |
CH3COO- |
| Carbonate |
CO32- |
| Phosphate |
PO43- |
| Hydronium |
H3O+ |
| Ammonium |
NH4+ |
| Sulfate |
SO42- |
Table of common
polyatomic cations, arranged by family. Alternate names are given in italics.
|
carbon |
nitrogen |
sulfur |
chlorine |
|
|
|
|
CO32- |
carbonate |
|
|
|
|
|
|
|
|
|
|
HCO3- |
hydrogen carbonate
(bicarbonate) |
|
|
|
|
|
|
SO42- |
sulfate |
|
SO32- |
sulfite |
|
|
|
|
S2O32- |
thiosulfate |
|
HSO4- |
hydrogen sulfate
(bisulfate) |
|
HSO3- |
hydrogen sulfite
(bisulfite) |
|
|
ClO4- |
perchlorate |
|
ClO3- |
chlorate |
|
ClO2- |
chlorite |
|
ClO- |
hypochlorite |
|
|
phosphorus |
cyanide |
cations |
metal oxyanions |
|
PO43- |
phosphate |
|
HPO42- |
hydrogen phosphate |
|
H2PO4- |
dihydrogen phosphate |
|
|
CN- |
cyanide |
|
OCN- |
cyanate |
|
SCN- |
thiocyanate |
|
|
|
CrO42- |
chromate |
|
Cr2O72- |
dichromate |
|
MnO4- |
permanganate |
|
|
oxygen |
organics |
|
OH- |
hydroxide |
|
O22- |
peroxide |
|
|
|
 |
If
you can remember the formula of the ion whose name ends with ate,
you can usually work out the formulas of the other family members
as follows:
|
modify stem name with: |
meaning |
examples |
|
-ate |
a common form, containing oxygen |
chlorate, ClO3-
nitrate, NO3-
sulfate, SO42- |
|
-ite |
one less oxygen than -ate form |
chlorite, ClO2-
sulfite, SO32-
nitrite, NO2-
|
|
per-, -ate |
same charge, but contains one more oxygen than -ate form |
perchlorate, ClO4-
perbromate, BrO4-
|
|
hypo-, -ite |
same charge, but contains one less oxygen than the -ite
form |
hypochlorite, ClO-
hypobromite, BrO- |
|
thio- |
replace an O with an S |
thiosulfate, S2O32-
thiosulfite, S2O22- |
Some
anions can capture hydrogen ions. For example, carbonate (CO32-
can capture an H+ to produce hydrogen carbonate HCO3-
(often called bicarbonate). Each captured hydrogen neutralizes one minus
charge on the anion.
|
modify stem name with: |
meaning |
examples |
|
hydrogen
or bi- |
(1) captured H+ ions |
hydrogen carbonate, HCO3- (a.k.a. bicarbonate)
hydrogen sulfate, HSO4- (a.k.a. bisulfate) |
|
dihydrogen |
(2) captured H+ ions |
dihydrogen phosphate, H2PO4-
|
|
Table of common
polyatomic cations, arranged by charge. Alternate names are given in italics. Select the
name of the ion for information about its occurrence, uses, properties, and
structure.
|
+2 |
|
Hg22+ |
mercury(I) or mercurous |
|
 |
|
+1 |
|
NH4+ |
ammonium |
|
H3O+ |
hydronium |
|
|
-1 |
|
C2H3O2- |
acetate |
|
ClO3- |
chlorate |
|
ClO2- |
chlorite |
|
CN- |
cyanide |
|
H2PO4- |
dihydrogen phosphate |
|
HCO3- |
hydrogen carbonate
or bicarbonate |
|
HSO4- |
hydrogen sulfate
or bisulfate |
|
OH- |
hydroxide |
|
ClO- |
hypochlorite |
|
NO3- |
nitrate |
|
NO2- |
nitrite |
|
ClO4- |
perchlorate |
|
MnO4- |
permanganate |
|
SCN- |
thiocyanate |
|
|
-2 |
|
CO32- |
carbonate |
|
CrO42- |
chromate |
|
Cr2O72- |
dichromate |
|
HPO42- |
hydrogen phosphate |
|
O22- |
peroxide |
|
SO42- |
sulfate |
|
SO32- |
sulfite |
|
S2O32- |
thiosulfate |
|
 |
|
-3 |
|
PO43- |
phosphate |
|
|
-1 CHARGE |
-2 CHARGE |
-3 CHARGE |
-4 CHARGE |
|
ion |
name |
ion |
name |
ion |
name |
ion |
name |
|
H2PO3- |
dihydrogen phosphite |
HPO32- |
hydrogen phosphite |
PO33- |
phosphite |
P2O74- |
pyrophosphate |
|
H2PO4- |
dihydrogen phosphate |
HPO42- |
hydrogen phosphate |
PO43- |
phosphate |
|
|
|
HCO3- |
hydrogen carbonate |
CO32- |
carbonate |
PO23- |
hypophosphite |
|
|
|
HSO3- |
hydrogen sulfite |
SO32- |
sulfite |
AsO33- |
arsenite |
|
|
|
HSO4- |
hydrogen sulfate |
SO42- |
sulfate |
AsO43- |
arsenate |
|
|
|
NO2- |
nitrite |
S2O32- |
thiosulfate |
|
|
|
|
|
NO3- |
nitrate |
SiO32- |
silicate |
|
|
|
|
|
OH- |
hydroxide |
C22- |
carbide |
|
|
|
|
|
CH3COO- |
acetate |
C2O42- |
oxalate |
|
|
|
|
|
CrO2- |
chromite |
CrO42- |
chromate |
|
|
|
|
|
CN- |
cyanide |
Cr2O72- |
dichromate |
|
|
|
|
|
CNO- |
cyanate |
C4H4O62- |
tartrate |
|
|
|
|
|
CNS- |
thiocyanate |
MoO42- |
molybdate |
|
|
|
|
|
O2- |
superoxide |
O22- |
peroxide |
|
|
|
|
|
MnO4- |
permanganate |
S22- |
disulfide |
|
|
|
|
|
ClO- |
hypochlorite |
|
|
|
|
|
|
|
ClO2- |
chlorite |
|
|
|
|
|
|
|
ClO3- |
chlorate |
|
|
|
|
|
|
|
ClO4- |
perchlorate |
|
|
|
|
|
|
|
BrO- |
hypobromite |
|
|
|
|
|
|
|
BrO2- |
bromite |
|
|
|
|
|
|
|
BrO3- |
bromate |
|
|
|
|
|
|
|
BrO4- |
perbromate |
|
|
|
|
|
|
|
IO- |
hypoiodite |
|
|
|
|
|
|
|
IO2- |
iodite |
|
|
|
|
|
|
|
IO3- |
iodate |
|
|
|
|
|
|
|
IO4- |
periodate |
|
|
|
|
|
|
|
AlO2- |
aluminate |
|
|
|
|
|
|
|
N3- |
azide |
|
|
|
|
|
|
You might be from the Northwest if you...
Know the state flower (Mildew).
Feel guilty throwing aluminum cans or paper in the trash.
Use the statement "sun break" and know what it means.
Know more than 10 ways to order coffee.
Know more people who own boats than air conditioners.
Feel overdressed wearing a suit to a nice restaurant.
Stand on a deserted corner in the rain waiting for the "WALK" signal.
Consider that if it has no snow or has not recently erupted, it is not a real mountain.
Can taste the difference between Starbucks, Seattle's Best, and Veneto's.
Know the difference between Chinook, Coho, and Sockeye salmon.
Know how to pronounce Sequim, Puyallup, Issaquah, Oregon, and Willamette.
Consider swimming an indoor sport.
In winter, go to work in the dark and come home in the dark, while only working eight-hour days.
Never go camping without waterproof matches and a poncho.
Are not fazed by "Today's forecast: showers followed by rain," and "Tomorrow's forecast: rain followed by showers."
Cannot wait for a day with "showers and sun breaks."
Have no concept of humidity without precipitation.
Know that Boring is a town in Oregon and not just a state of mind.
Can point to at least two volcanoes, even if you cannot see through the cloud cover.
Notice "the mountain is out" when it is a pretty day and you can actually see it.
Put on your shorts when the temperature gets above 50, but still wear your hiking boots and parka.
Switch to your sandals when it gets about 60, but keep the socks on.
Have actually used your mountain bike on a mountain.
Think people who use umbrellas are either wimps or tourists.
Knew immediately that the view out of Frasier's window was fake.
Buy new sunglasses every year, because you can't find the old ones after such a long time.
Measure distance in hours.
Often switch from "heat" to "a/c" in the same day.
Use a down comforter in the summer.
Design your kid's Halloween costume to fit over a raincoat.
Know all the important seasons: Almost Winter, Winter, Still Raining (Spring), Road Construction (Summer), Deer & Elk season (Fall).
Actually understand these jokes and send them to all your friends in the northwest or those who used to live here!

Tables of lots of polyatomic ions
| The 9 polyatomic ions to know and write on your notecard: |
| Name |
Charge |
Formula |
| Hydroxide |
1- |
OH- |
| Cyanide |
1- |
CN- |
| Nitrate |
1- |
NO3- |
| Acetate |
1- |
CH3COO- |
| Carbonate |
2- |
CO32- |
| Phosphate |
3- |
PO43- |
| Hydronium |
1+ |
H3O+ |
| Ammonium |
1+ |
NH4+ |
| Sulfate |
2- |
SO42- |
Balancing reactions:


Honda Designs Car Friendly for Dogs
Sample Standard Enthalpies of Formation Table--See Table 6.2 in your text: