Chemistry 121 Fall 2003 Oregon State University
Exam 1 October 23, 2003 Dr. Jennifer Travers
Dr. Richard Nafshun
DO NOT OPEN THIS EXAM UNTIL INSTRUCTED.
CALCULATORS ARE NOT TO BE SHARED.
Instructions: You should have with you several number two pencils, an eraser, your 3" x 5" notecard, and your University ID Card. You may use a basic calculator (for example, TI-25X Solar or TI-30XA) if you wish. If you have notes or electronic devices with you, place them in a sealed backpack and place the backpack OUT OF SIGHT. Or place the notes directly on the table at the front of the room.
Fill in the front page of the Scantron answer sheet with your last name, first name, middle initial, and student identification number. Leave the class section number blank. Enter the following test form number:
This exam consists of 25 multiple-choice questions. Each multiple-choice question has four points associated with it. Select the best multiple-choice answer by filling in the corresponding circle on the rear page of the answer sheet. If you have any questions before the exam, please ask. If you have any questions during the exam, please raise your hand to attract the attention of a proctor. The proctor will come to you. Open and start this exam when instructed. Present your ID card when submitting the exam. Place your 3" x 5" notecard in the appropriate stack. You may keep the multi-choice portion of this exam, so please mark the answers you selected on it.
Avogadro’s Number = 6.02 x 1023
1. How many milliseconds are in 12.5 hours?
(A) 1.25 x 10-9 ms
(B) 3.47 x 10-6 ms
(C) 1.25 x 104 ms
(D) 4.50 x 107 ms
(E) 45.0 ms
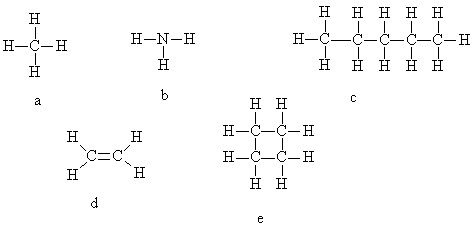
2. Which of the following drawings represents the structure of pentane?

3. Consider the following operation 0.0450 x 192.1 = ? Which answer below contains the correct number of significant figures?
(A) 8.6445
(B) 8.645
(C) 8.64
(D) 8.6
(E) 9
4. Which of the following is an element?
(A) carbon dioxide
(B) plastic
(C) sodium
(D) hexane
5. A piece of silver with a total mass of 12.31 g was placed in a graduated cylinder filled with 10.00 mL of water. The density of silver is 10.50 g/mL. What will be the final volume in the graduated cylinder?
(A) 22.81mL
(B) 11.17 mL
(C) 22.31 mL
(D) 10.85 mL
(E) 9.33 mL
6. Which of the following chemical formulas does not exist?
(A) MgI2
(B) KNO3
(C) CaS
(D) LiCN
(E) Al3O2
7. Consider the ionic compound potassium phosphate. How many of each type of atom are present in one potassium phosphate unit?
(A) 12 K, 4P, 4O
(B) 3 K, 4P, 4O
(C) 12 K, 4P, 12O
(D) 3 K, 1P, 4O
(E) 7 K, 1P, 12O
8. What is the proper name for the formula P4S3?
(A) pentaphosphorous trioxide
(B) tetrasulfurous phosphide
(C) tetraphosphorous trisulfide
(D) potassium trisulfide
(E) butylphosphorous propylsulfide
9. Which is the correct formula for lithium nitride?
(A) LiN3
(B) Li3N
(C) LiNH3
(D) Li2NO3
(E) Li2N3
10. Which of the following is considered a molecule?
(A) CuCl2
(B) K3PO4
(C) NaBr
(D) CH4
(E) LiNO3
11. Which of the following statements is FALSE?
(A) An electron is located outside of the nucleus.
(B) An electron has an approximate mass of 1 atomic mass unit.
(C) An electron has a charge of -1.
(D) A cation has less electrons than protons.
(E) A neutral atom has an equal number of protons and electrons.
12. What does the following symbolism represent: 57Fe 2+ ?
(A) An iron atom with 26 protons, 24 electrons, and 24 neutrons
(B) An iron atom with 24 protons, 26 electrons, and 31 neutrons
(C) An iron atom with 28 protons, 26 electrons, and 29 neutrons
(D) An iron atom with 26 protons, 24 electrons, and 31neutrons
(E) An iron atom with 24 protons, 31 electrons, and 33 neutrons
13. Consider atoms X and Z. Choose which statement best describes the relationship between atoms X and Z.
Atom X- has 12 protons, 12 electrons and 14 neutrons
Atom Z- has 12 protons, 12 electrons and 13 neutrons
(A) Atom X and atom Z are different ions of the same element.
(B) Atom X and atom Z are different elements with the same charge.
(C) Atom X and atom Z are isotopes of the same element.
(D) Atom X and atom Z are different elements with different charges.
14. Which of the following atoms is least likely to form a positively charged ion?
(A) Sodium
(B) magnesium
(C) cobalt
(D) silver
(E) chlorine
15. How many moles of zinc are present in 8.34 x 10-7 g of zinc?
(A) 5.37 x 103 moles
(B) 7.20 x 10 29 moles
(C) 1.30 x 10-8 moles
(D) 7.72 x 107 moles
(E) 1.39 x 10-30 moles
16. How many hydrogen atoms are present in 1.00 mole of water?
(A) 2.00
(B) 18.0
(C) 6.02 x1023
(D) 1.20 x 1046
(E) 1.20 x 1024
17. There are 4.20 x 1021 valium molecules in 2.00 g of valium. What is the molar mass of valium?
(A) 392 g/mol
(B) 286 g/mol
(C) 840 g/mol
(D) The answer cannot be calculated without the molecular formula of valium.
18. Chlorine has two naturally occurring isotopes. 35Cl has a mass of 34.97 g/mol and is 75.47% abundant. 37Cl has a mass of 36.97 g/mol and is 24.53% abundant. What is the average atomic mass of chlorine?
(A) 26.41 g/mol
(B) 35.97 g/mol
(C) 35.46 g/mol
(D) 71.94 g/mol
19. Which of the following compounds has the greatest percent nitrogen?
(A) NaNO3
(B) NaNO2
(C) NH4NO2
(D) NO
20. What is the % composition (by mass) of phosphorous in phosphoric acid (H3PO4)?.
(A) 31.6 % P
(B) 65.3 % P
(C) 58.1% P
(D) 30.0 % P
(E) 17.6% P
21. Provide the coefficients needed to balance the following equation:
a C3H8 + b O2 ŕ c CO2 + d H2O
(A) a=1, b=2, c=2, d=3
(B) a=1, b=5, c=3, d=4
(C) a=2, b=3, c=2, d=3
(D) a=2, b=3, c=1, d=6
(E) a=3, b=2, c=2, d=3
22. Consider the following reaction:
4 P + 5 O2 ŕ P4O10
How many moles of phosphorus are required to produce 3.00 moles of P4O10?
(A) 0.500 mol P
(B) 1.50 mol P
(C) 3.00 mol P
(D) 6.00 mol P
(E) 12.0 mol P
23. Shown below is the balanced equation for the combustion of methane. Assuming that oxygen is in excess, what is the theoretical yield of H2O from the combustion of 12.5 g of methane?
CH4 + 2 O2 ŕ CO2 + 2 H2O
(A) 1 g
(B) 28.1 g
(C) 12.5 g
(D) 25.0 g
24. Consider the following reaction:
2 Na + 2 H2O ŕ 2 NaOH + H2
In a given experiment, the theoretical yield of hydrogen gas for the above reaction is 7.00g. If the reaction actually produces 2.50 g hydrogen gas, what is the percent yield for the reaction?
(A) 0.50 %
(B) 2.80 %
(C) 17.7%
(D) 50.0 %
(E) 35.7%
25. A student places 10.0 g of sodium bromide in a volumetric flask and fills the flask with water to a final volume of 50.0 mL. What is the molarity of the resulting solution?
(A) 1.94 M
(B) 4.85 x 10-3 M
(C) 51.5 M
(D) 0.200 M